 点击上方“知识城邦”关注我们吧!
点击上方“知识城邦”关注我们吧!
前言各位好!真实世界数据、真实世界证据RWD,听起来总是很耳熟,但究竟在肿瘤学研究中应用的细节上与RCT有哪些分别呢?在真实世界研究中不同领域的专家有哪些小的心得体会?具体能够为后来人做标杆的RWS又有哪些呢?ps.本号分享的真实世界内容可以在公众号内搜索RWD/RWS。
今日迎接大佬,就不深入展开了,笔记里会结合国内外例子,与大家分享一系列真实世界研究的案例。
本 文 约3056字 多图预警
认真阅读 需 要 5-10 minReal-world data in oncology: a questionnaire-based analysis of the academic research landscape examining the policies and experiences of the cancer cooperative groups
R. Saesen1,2*, D. Lacombe1y & I. Huys2y
ESMO Open 21 February 2023
Objectives: Real-world data (RWD) have quickly emerged as an important source of information to address uncertainties about new treatments, including novel anticancer therapies. Many stakeholders are using such data and the evidence derived therefrom to answer the questions that remain about the safety and effectiveness of antitumor medicines after their approval by regulators. Our objective was to investigate the academic RWD study landscape and explore to what extent RWD are being integrated into investigator-initiated clinical research.目的:真实世界数据方兴未艾,也是一种能回答抗肿瘤治疗新方式临床应用困惑点的重要方法。许多机构正在基于真实世界数据和由此得出的证据来回答监管机构批准抗肿瘤药物后仍然存在的关于安全性和有效性的问题。本研究的目标是调查学术领域内RWD的研究概况,并探索RWD在多大程度上被整合到研究者发起的临床研究中。
Methods: We designed an online survey that was distributed between May and August 2022 to representatives of cancer cooperative groups active in Europe, North America, South America, Asia, and/or Oceania.
方法:我们设计了一项在线调查,在2022年5月至8月期间分发给活跃在欧洲、北美、南美、亚洲和/或大洋洲的癌症合作组织的代表。
Results: In total, 125 cooperative groups operating in 58 different countries and conducting research across 13 distinct cancer domains participated in the survey. While most of the responders (67.2%) did not have a formal policy in place to gather and utilize RWD, a majority (68.0%) had carried out studies involving the analysis of such data before, both for exploratory and confirmatory purposes. The groups that were experienced in capturing and interpreting RWD had mainly worked with observational RWD that were not predominantly prospective or retrospective in nature and which originated from disease registries, electronic health records, and patient questionnaires. They perceived the low costs and the large scale of RWD research to be its most significant benefits, and viewed the accompanying methodological and operational challenges as its biggest constraints. However, they did not have a common understanding of what RWD were. Despite their experience with analyzing RWD, their research portfolio still primarily comprised traditional clinical trials; 62.5% of the groups that had never undertaken any RWD studies were nonetheless planning to initiate them in the future.结果:调查显示共有125个合作小组在58个不同的国家,13个不同的癌症领域进行研究的机构代表,参与了这项调查。虽然大多数回复者(67.2%)没有正式的政策来收集和利用RWD,但大多数(68.0%)曾开展过涉及分析此类数据的研究,既有探索性的,也有验证性的。在收集和解释RWD方面经验丰富的小组主要与观察性RWD合作,这些RWD性质上不完全是前瞻性或回顾性的,数据源来自疾病登记、病历记录和患者问卷。他们认为低成本和大样本量的RWD研究是这个方向的优势,并将随之而来的方法学和领域内挑战被视为其最大的制约因素。然而,他们对什么是RWD并没有统一的理解。尽管他们有分析RWD的经验,但他们的研究组合仍然主要包括传统的临床试验;62.5%的从未进行过RWD研究的小组仍然计划在未来启动这些研究。
Conclusions: Cancer cooperative groups are already incorporating RWD studies into their research agendas, but still lack knowledge and expertise in this regard, and do not agree on what RWD are. The conduct of conventional clinical trials continues to be their priority.
结果:癌症合作组织已经将RWD研究纳入他们的研究议程,但仍然缺乏这方面的知识和专业知识,并且对RWD是什么没有达成一致。进行常规临床试验仍然是他们的优先事项。
Keywords: real-world data; real-world evidence; oncology; cancer; cooperative groups; survey
Highlights
- RWD are increasingly being used to support the development and market access of anticancer therapies.
- Cancer cooperative groups around the world are already incorporating RWD studies into their research agendas.
- They have mainly used observational RWD drawn from cancer registries, electronic health records, and patient questionnaires.
- Nevertheless, they continue to prioritize the conduct of traditional clinical trials.
- There is still room for expanding the role of academia in interventional RWD research.
RWD正越来越多地被用于支持抗肿瘤治疗方法的开发和市场准入。
世界各地的癌症合作组织已经将RWD研究纳入他们的研究议程。
他们主要使用从癌症登记、电子健康记录和患者问卷中提取的观察性RWD。
尽管如此,他们仍然优先进行传统的临床试验。
干预性RWD研究在学术界仍然大有可为。
学习笔记
1.为什么要提真实世界呢?因为RCT太贵了,太烧钱了。
出资人觉得劳资花了这么多钱,就搞了一个这?太对不起money了吧?
CRO觉得这文件签署的行政负担也太重了吧,到处找大夫签字。医生觉得我就想设计个研究,搞这么复杂干嘛。说实话临床试验我们国家走得不如欧美,你看欧洲这也被不厌其烦地行政审核搞头大了,说我们能不能简洁一些。
不缺钱不缺粮,当然RCT好啊。但组办太多RCT的人也发现,RCT的行政成本蛮高的。而且也贼烦。不是我这么说,(我----李云龙的小弟,李小龙的迷弟,怎么可能做得起RCT呢?)老牌欧美的学者专门发文写了这个问题.....真实世界为什么需要新瓶装旧酒呢?监管对于这个换了瓶子的酒怎么看呢??是学术套路?还是一片蓝海?可能每个人心中都有答案吧。
嗯上面这些西文李云龙肯定看不懂,但看了这个肯定想要喊和尚~!开炮!
SupplementaryFigure 1: Standard delegation log.
签字怎么这么乱。。。。
Supplementary Figure 2: Instructions to reset a password and to unlock an account in an online platform for clinical trials (anonymized).一堆重置密码
Supplementary Figure 3: Mandatory online courses to gain access to an online platform.
Only one of these (applying signatures) refers to a function that is performed by the principal investigator (PI). Yet the PI is required to complete all of the courses and must pass an exam for each one of them.
Annex 1 (Online access): Proposed simplified Delegation log template.
10.1016/j.annonc.2022.09.162
2.我们国家地大物博人口众多,真实世界数据对于肿瘤研究也至关重要。大数据提了这么久,现在元宇宙、云计算都出来了,只有大样本有效量的积累才能够做精品,而不是简单的模仿。我们国家的NCDB大样本数据库,翘首以盼啊。
远的不说就新辅助食管癌后哪些患者需要做辅助治疗,Annals of surgery上就有一篇经典范文,也是真实世界数据处理的典范。就问国内几个人能把代码复现一下?天天搞套路,别说外国人,咱自己人看了都烦。
这个研究调查的范围很窄,连东方大国、西方大国的著名学会都没调查都敢瞎在自己的一亩三分地上“吹牛”。有一说一当年列强的后代真傲慢。他倒是来学中文,去CDE官网好好看看啊。这也提醒了我自己要多读多学。
这篇文献的参考文献倒是引用的很精华,附件也可以以及认识认识这些欧洲小国肿瘤研究圈子里的一些乱七八糟的协会。
3.光讲理论了,这里写下同期配发的典型案例——胰腺癌的一线化疗方案。这个研究的CRF表蛮有借鉴意义的。推荐大家看一下。真实世界数据最大的难点就是数据异质性和分析处理。AI相关的一些方法还是要多学习的。
10.1126/scitranslmed.abn6911
10.1016/j.esmoop.2022.100773
10.1016/j.esmoop.2022.100777
为学不读CRF,便是英雄也枉然!
目录
1. INTRODUCTION
2. Materials and Methods
3. Results(Supplementary Material)
3.1 Demographic characteristics of the survey sample(Figure 1)
3.2 Policies and experiences with regard to RWD(Figure 2)(Table 1)
3.3 Understanding, opportunities, challenges, and applications of RWD(Figure 3)
4. Discussion
前几天看了介绍王坚院士的视频,收获很大。放在这个不严谨的地方供大家学习。
 </p>
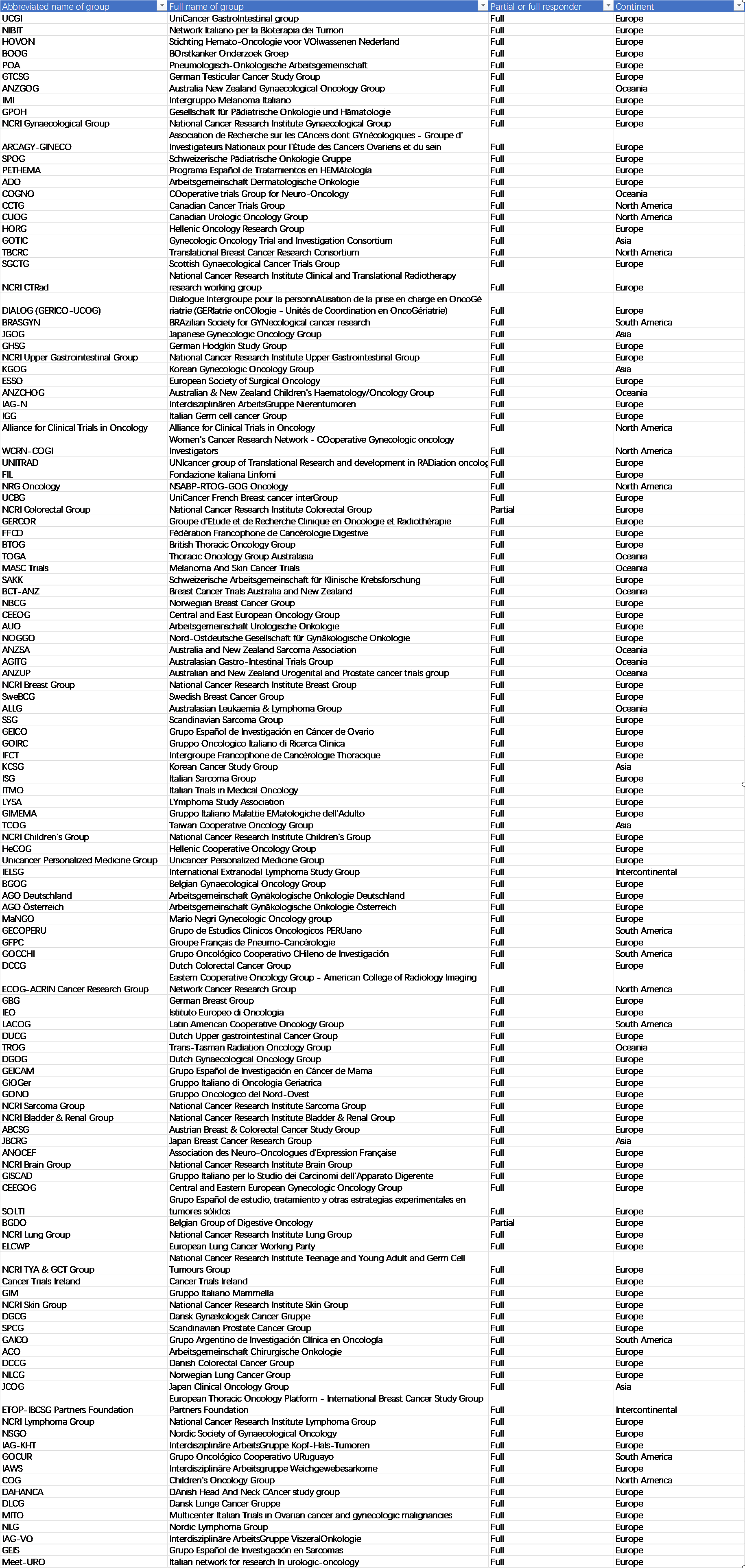
</p>眼尖的在上图找找,台湾永远是中国的,欧洲弹丸小国,格局和视野确实不行啊!
调查的鄙视链,你看美国ASCO、AACR等主流学会也不理欧洲人哈哈。
问了哪些问题呢?
Figure 1. Demographic characteristics.
Breakdown of the participating groups by (A) the oncological domains in which they conducted research, (B) the types of anticancer therapies they investigated, (C) the countries in which they were active, and (D) the number of institutions they comprised. The numbers reported for (A), (B), and (C) exceed the total number of responders upon aggregation because multiple response options could be selected for the matching questions.最后在这里对文章中没有把我国癌症研究组纳入调查范围且将台湾单列出来的行为表示鄙视。我们国家的专家学者将在不远的将来,向世界文明证实,缺席中国的真实世界研究,苍白无色!
Figure 2. Experience with real-world data (RWD) studies and sources.
Experience of the participating groups with (A) RWD studies in general, (B) different sources of RWD, (C) specific types of RWD studies, and (D) RWD studies as compared with traditional randomized controlled trials (RCTs), expressed as a proportion of the total number of studies they had carried out.The data shown for (B), (C), and (D) were obtained from a subset of the responders that had worked with RWD before.For (B), (C), and (D), the numbers in brackets indicate the absolute number of groups corresponding with each percentage.The numbers reported for (B) exceed the total number of responders upon aggregation because multiple response options could be selected for the matching question.
Table 1. Examples of real-world data (RWD) studies.Selected examples cited by the participating cooperative groups of RWD studies that they had initiated
Figure 3. Understanding of real-world data (RWD).
Participating groups’ understanding of RWD, categorized based on the classification system of Makady et al.1 The data shown were obtained from a subset of the responders that had worked with RWD before. The percentages displayed do not add up to exactly 100% due to rounding. RCT, randomized controlled trial.

大展宏兔~! |