马上注册,阅读更多内容,享用更多功能!
您需要 登录 才可以下载或查看,没有账号?立即注册 
×
纳入了840例完整手术切除的Ⅰb-Ⅲa期NSCLC患者,随机分为长春瑞滨+顺铂辅助化疗组和对照组。与JBR10试验结果相似,化疗组能够耐受化疗药物的毒性反应,中位随访76个月,化疗组和对照组的中位OS为65.7和43.7个月。辅助化疗可明显提高NSCLC根治术后Ⅱ期和Ⅲa期患者的7年生存率(8.4%,P=0.013),但Ⅰ期患者无法从化疗中获益。
纳入来自14个国家101个中心的840例IB-IIIA期(1986版TNM分期)NSCLC患者,完全切除后随机接受长春瑞滨联合顺铂(NP组,30mg/m2,100mg/m2 ,n=407)辅助治疗或不接受辅助治疗(n=433)。主要研究终点是意向治疗(ITT)人群的OS,次要研究终点是无病生存期(DFS)和安全性。
最终研究结果显示,NP组和观察组的中位OS分别是65.7个月和43.7个月,HR=0.80(95%CI 0.66-0.96,P=0.017),OS有显著的统计学差异,1年OS率、2年OS率、5年OS率和7年OS率分别是2.8%、4.7%、8.6%和8.4%。NP组和观察组的中位DFS分别为36.3个月和20.7个月,HR=0.76(95% CI 0.64-0.91,P=0.002),DFS有显著的统计学差异,6个月、1年、2年、5年和7年的DFS率分别为9%、9.5%、9.6%、8.7%和5.5%。NP辅助化疗最常见的3-4级血液学毒性是中性粒细胞减少、贫血和FN。其他常见的非血液学毒性包括虚弱、恶性呕吐、厌食和感染。
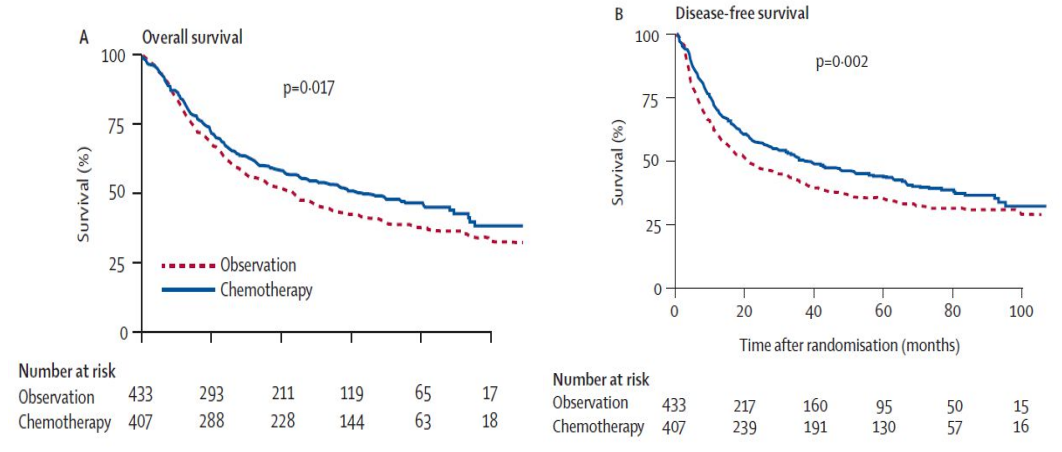
Background: Whether adjuvant chemotherapy improves survival of patients with non-small-cell lung cancer (NSCLC) is not known. We aimed to compare the effect of adjuvant vinorelbine plus cisplatin versus observation on survival in patients with completely resected NSCLC. Methods: 840 patients with stage IB-IIIA NSCLC from 101 centres in 14 countries were randomly assigned to observation (n=433) or to 30 mg/m(2) vinorelbine plus 100 mg/m(2) cisplatin (n=407). Postoperative radiotherapy was not mandatory and was undertaken according to every centre's policy. The primary endpoint was overall survival. Analysis was by intention to treat. This trial is registered as an International Standard Randomised Controlled Trial, number ISRCTN95053737. Findings: 367 patients in the chemotherapy group and 431 in the control group received their assigned treatment. 301 (36%) patients had stage IB disease, 203 (24%) had stage II disease, and 325 (39%) had stage IIIA disease. Tolerance to chemotherapy mainly included neutropenia in 335 (92%) patients and febrile neutropenia in 34 (9%); seven (2%) toxic deaths were also recorded. Compliance was greater with cisplatin than with vinorelbine (median dose intensity 89% [range 17-108] vs 59% [17-100]). After a median follow-up of 76 months (range 43-116), median survival was 65.7 months (95% CI 47.9-88.5) in the chemotherapy group and 43.7 (35.7-52.3) months in the observation group. Adjusted risk for death was significantly reduced in patients assigned chemotherapy compared with controls (hazard ratio 0.80 [95% CI 0.66-0.96]; p=0.017). Overall survival at 5 years with chemotherapy improved by 8.6%, which was maintained at 7 years (8.4%). Interpretation: Adjuvant vinorelbine plus cisplatin extends survival in patients with completely resected NSCLC, better defining indication of adjuvant chemotherapy.
参考文献:
- Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR, Le Groumellec A, Lorusso V, Clary C, Torres AJ, Dahabreh J, Souquet PJ, Astudillo J, Fournel P, Artal-Cortes A, Jassem J, Koubkova L, His P, Riggi M, Hurteloup P. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006 Sep;7(9):719-27. doi: 10.1016/S1470-2045(06)70804-X. Erratum in: Lancet Oncol. 2006 Oct;7(10):797. PMID: 16945766.
|