马上注册,阅读更多内容,享用更多功能!
您需要 登录 才可以下载或查看,没有账号?立即注册 
×
Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, Postmus P, Collette L, Musat E, Senan S; EORTC Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007 Aug 16;357(7):664-72. doi: 10.1056/NEJMoa071780. PMID: 17699816.
Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, Keijser A, Faivre-Finn C, Senan S. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015 Jan 3;385(9962):36-42. doi: 10.1016/S0140-6736(14)61085-0. Epub 2014 Sep 14. Erratum in: Lancet. 2015 Jan 3;385(9962):28. PMID: 25230595.
The EORTC performed this landmark randomized trial to evaluate the role of prophylactic cranial irradiation in patients with extensive-stage small cell lung cancer who experienced any response to systemic chemotherapy. The study allowed various radiation therapy fractionation schedules including 20 Gy in 5 or 8 fractions, 24 Gy in 12 fractions, 25 Gy in 10 fractions, or 30 Gy in 10 or 12 fractions. The study did not mandate brain imaging as part of staging unless symptoms suggested the presence of brain metastasis. The primary endpoint was the development of symptomatic brain metastasis. The study randomized 286 patients between 2001 and 2006.
Key Point The incidence of symptomatic brain metastasis was significantly reduced in the prophylactic cranial irradiation arm from 41.3% to 16.8% (HR = 0.27, 95% CI 0.16 to 0.44, P < 0.001; see Table 3.12).
Key Point Patients randomized to receive prophylactic cranial irradiation experienced improved overall survival with median survival of 6.7 versus 5.4 month (HR = 0.68, 95% CI 0.52 to 0.88, P = 0.003). The rate of 1-year survival was 27.1% versus 13.3% in favor of the prophylactic cranial irradiation group.
Slotman et al. concluded that “prophylactic cranial irradiation should be part of standard care for all patients with small cell lung cancer who have a response to initial chemotherapy.” One potential criticism of this study is that standard brain imaging in asymptomatic patients was not part of staging workup, so it is unclear how many patients who underwent prophylactic cranial irradiation may have had asymptomatic brain metastasis.
It is also important to note that a recent phase 3 prospective randomized trial performed at 47 institutions in Japan evaluated the role of prophylactic cranial irradiation in patients with extensive-stage small cell lung cancer who experienced a response to platinum-based doublet chemotherapy (Takahashi et al. Lancet Oncology 18(5), 2017). In this trial, the addition of prophylactic cranial irradiation did not improve overall survival compared with observation in this patient population. Patients enrolled onto this trial by Takahashi et al. did have baseline brain imaging to ensure there was no brain metastasis on MRI prior to enrollment on the study.
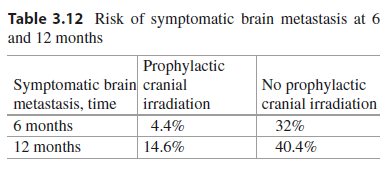
Table 3.12 Risk of symptomatic brain metastasis at 6 and 12 months
| Symptomatic brain metastasis, time | Prophylactic cranial irradiation |
No prophylactic cranial irradiation
| | | | | | |
Slotman B, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomized controlled trial. Lancet. 2015;385:36–42.
This landmark trial built on the results of the Jeremic et al. study in patients with extensive-stage small cell lung cancer by randomizing patients with any response to chemotherapy to either prophylactic cranial irradiation alone or prophylactic cranial irradiation and consolidative radiation therapy to the site of thoracic disease.
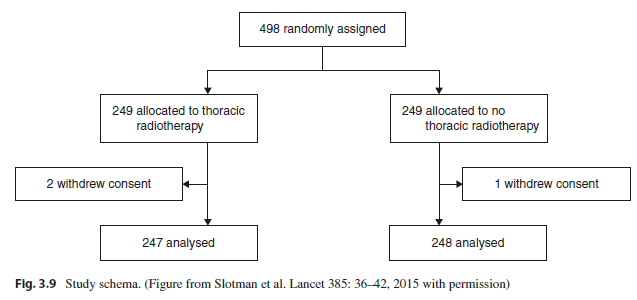
Between 2009 and 2012, this CREST trial randomized 498 patients who experienced any response to a cisplatin and etoposide combination of chemotherapy to either prophylactic cranial irradiation or prophylactic cranial irradiation and 30 Gy in 10 fractions of thoracic radiation therapy (Fig. 3.9). The primary endpoint was overall survival at 1 year.
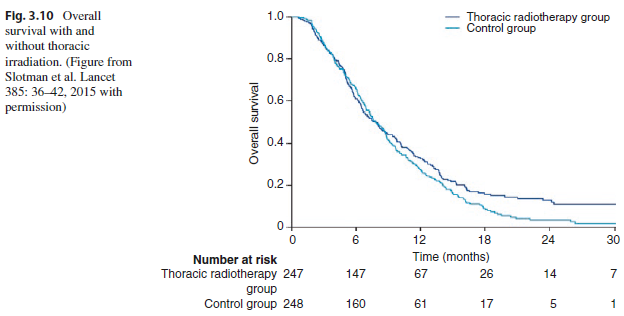
Key Point There was no statistically significant difference in 1-year overall survival between the two arms of the trial (33% with and 28% without thoracic radiation, HR = 0.84, 95% CI 0.69 to 1.01, P = 0.066). However, there was a significant improvement in 2-year overall survival with the addition of thoracic radiation therapy (13% versus 3%, P = 0.004). The number needed to treat with radiation therapy to the thorax to avoid one death was 10.6 (Fig. 3.10).
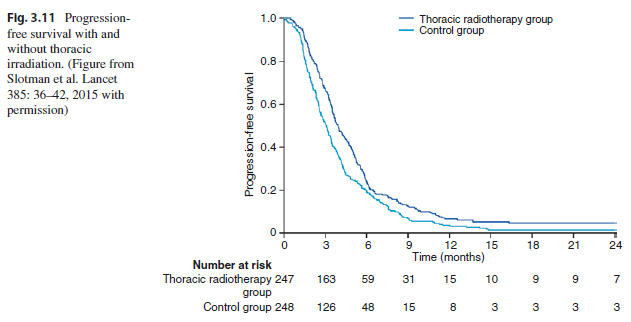
Key Point The addition of radiation therapy to the thorax also improved progression-free survival (HR = 0.73, 95% CI 0.61 to 0.87, P = 0.001). The 6-month rate of progression-free survival was 24% versus 20%.
The thorax was the first site of progression of disease in 41.7% versus 77.8% of patients, favoring the group of patients who received thoracic radiation therapy (P = 0.009; see Fig. 3.11).
There were no severe acute or late toxic effects recorded for thoracic radiotherapy. Thoracic radiation therapy was well tolerated with 95% of patients completing radiation therapy as specified by the protocol.
This landmark randomized trial demonstrated a survival improvement at 2 years and improved progression-free survival in patients with extensive-stage small cell lung cancer who experienced any response to systemic therapy and then went on to receive thoracic radiation therapy compared with patients who did not receive thoracic radiation therapy. Slotman et al. stated that 40% of patients who received thoracic radiation therapy eventually experienced intrathoracic recurrence and they questioned whether more aggressive dosage and fractionation than what was used in this study should be investigated. Moreover, due to high rates of extrathoracic progression of disease, Slotman et al. called for further evaluation in trials of whether such patients would benefit from the addition of radiation therapy to select extrathoracic sites of disease.
RTOG 0937, which is discussed next in this chapter, was designed to evaluate whether radiation therapy to up to four sites of extrathoracic metastatic disease would improve outcomes in patients with extensive-stage small cell lung cancer who had responded well to initial therapy. In summary, Slotman et al. concluded from the results of this CREST trial that “thoracic radiotherapy should be considered for patients with extensive stage small-cell lung cancer who have responded to chemotherapy.”
|
|
