马上注册,阅读更多内容,享用更多功能!
您需要 登录 才可以下载或查看,没有账号?立即注册 
×
中国医师协会肿瘤消融治疗技术专家组 中国医师协会介入医师分会肿瘤消融专业委员会中国抗癌协会肿瘤消融治疗专业委员会 中国临床肿瘤学会消融专家委员会
摘要
局部热消融技术在肺部结节治疗领域正处在起步与发展阶段,为了肺结节热消融治疗的临床实践和规范发展,由“中国医师协会肿瘤消融治疗技术专家组”“中国医师协会介入医师分会肿瘤消融专业委员会”“中国抗癌协会肿瘤消融治疗专业委员会”“中国临床肿瘤学会消融专家委员会”组织多学科国内有关专家,讨论制定了“热消融治疗肺部亚实性结节专家共识(2021年版)”。主要内容包括:①肺部亚实性结节的临床评估;②热消融治疗肺部亚实性结节技术操作规程、适应证、禁忌证、疗效评价和相关并发症;③存在的问题和未来发展方向。
关键词:肺肿瘤;筛查;肺亚实性结节;肺磨玻璃结节;热消融
1. 前言
在世界范围内肺癌发病率虽位居第二,但死亡率仍高居首位[1],因此早期发现、早期诊断、早期治疗是降低肺癌死亡率的重要手段。2011年,美国国家肺癌筛查试验(National Lung Screening Trial, NLST)首次报告了低剂量螺旋计算机断层扫描(low-dose computed tomography,LDCT)筛查可以显著降低高危人群肺癌的死亡率,与标准胸部X线检查相比,LDCT筛查可使肺癌病死率降低20%[2]。近年来,随着LDCT筛查项目的广泛开展,越来越多的无症状肺结节被发现。肺结节在我国检出率为20%-80%[3-6],LDCT筛查97%以上的肺部结节为良性病变,肺癌的检出率仅为0.7%-2.3%[2,4-8]。过高的检出率可能导致过度诊断、过度治疗、浪费医疗资源及增加受检者焦虑心理[9-13]。目前针对肺结节的筛查和处理指南主要有:美国国家综合癌症网络(National Comprehensive Cancer Network,NCCN)、Fleischner学会、美国胸科医师协会(AmericanCollege of Chest Physicians, ACCP)、亚洲和中国[14,15]等指南,由于指南制定者的专业背景、所属国家或地域不同,至今未达成统一的共识。无论何种指南存在何种差异,最终对于肺结节处理原则是一致的:随访观察和外科手术切除。外科手术的进步,特别是电视辅助胸腔镜手术(video-assisted thoracoscopic surgery, VATS)的普遍应用,使得早期肺癌的治疗疗效、术后并发症和死亡率有了一定的改善[16-19],但是仍有许多问题需要解决。
肺部结节常被认为可能是癌前病变或早期肺癌的征象,而磨玻璃结节(ground-glass nodule,GGN或groundglass opacity,GGO)样肺癌具有“惰性”发展和极少有远处转移等特点,预后良好,手术切除后5年生存率可达100%[20-27],因此这类肺癌不同于“传统意义”上的早期肺癌,应该是肺癌中的特殊亚型。此类病变过早地应用VATS切除存在一定的问题:①肺结节尤其浸润前病变,过早的手术介入,会导致过早的器官损伤和肺功能损失,术后可能会出现各种并发症,而且早期手术与随访择期手术相比并不能显著改善患者总体生存期;②多发肺结节目前仍无明确的手术方式选择标准,也无剩余结节的后续处理原则;③术前肺结节的诊断是依赖影像学判断,无病理支持,对术前判断有风险的肺结节进行手术切除,术后可能证实为良性病变,使患者经历了不必要的手术和术后并发症[28-30];④随着人口的老龄化,越来越多的早期肺癌患者在75岁以上,这些患者往往无法选择手术治疗。另外,随访也存在问题:每次间隔多久随访?何时终结随访?对于受检者每一次复查都可能带来心理恐慌,甚至严重地影响受检者的生活质量[31],同时也增加了受检者的X射线暴露。为了克服上述问题,需要拓展处理肺结节的新方法。
局部热消融术作为一种精准的微创技术已经应用于早期肺癌的治疗,每年的治疗例数迅速增加[32-41],该技术具有创伤小、疗效明确、安全性高、可重复性强、适应人群广等特点。热消融技术在肺部结节治疗领域正处在起步与发展阶段[42-49],为了推动热消融技术在肺结节治疗中的合理运用,本着安全、有效、规范和可持续发展的原则,由“中国医师协会肿瘤消融治疗技术专家组”“中国医师协会介入医师分会肿瘤消融专业委员会”“中国抗癌协会肿瘤消融治疗专业委员会”“中国临床肿瘤学会消融专家委员会”组织多学科国内有关专家,讨论制定了《热消融治疗肺部亚实性结节专家共识(2021年版)》,以期为热消融治疗肺结节的临床实践和规范发展提供参考。
2. 肺结节的概念和分类
2.1 概念[50-54]
由于不同病因造成的肺泡内含气量减少、细胞数量增多、肺泡上皮细胞增生、肺泡间隔增厚和终末气囊内部分充血水肿,这种病理变化在肺部影像学上常表现为:局灶性、边界清楚或模糊、直径(或最大径)≤30 mm、圆形或类圆形、密度增高的阴影。可为单发或多发,不伴有肺不张、肺门及纵隔淋巴结肿大和胸腔积液。
2.2 分类
2.2.1 按病变性质[55]
①良性:良性肿瘤、各种感染性疾病、风湿类疾病、先天性疾病、肺出血等;②恶性:肺癌(浸润前病变、浸润性癌)、淋巴瘤、肉瘤、肺转移瘤等。
2.2.2 按密度
可分为实性和亚实性肺结节。①实性肺结节(solid nodule):CT肺窗观察,肺内圆形或类圆形的密度增高病变,可掩盖其内走行的血管和支气管,纵隔窗图像显示为软组织密度;②亚实性肺结节(subsolidnodule)[28,53,56,57]:CT肺窗观察,肺内圆形或类圆形的高密度病变,不掩盖其内走行的血管和支气管影,纵隔窗图像不显示,类似为磨玻璃样,因此称为GGN或GGO。亚实性肺结节又分为:纯GGN(pure GGN, pGGN)和磨玻璃密度中带有实性密度成分的混合性GGN(mixed GGN,mGGN),后者也称部分实性结节(partial solid nodule,PSN)。在亚实性肺结节中如果是潜在恶性或恶性其病理类型为肺腺癌相关的组织亚型[53,58-62],可涉及从肺泡上皮不典型腺瘤样增生(atypical adenomatous hyperplasia,AAH)到原位腺癌(adenocarcinoma in situ, AIS),到微浸润腺癌(microinvasive lung adenocarcinoma, MIA),再到浸润性腺癌(invasive adenocarcinoma, IAC)等多个腺癌演进阶段。本共识主要述及亚实性肺结节即GGN。
2.2.3 按大小[14,57]
①微小结节:直径<5 mm(体积<100 mm3);②小结节:直径5 mm-10 mm(体积100 mm3-400 mm3);③结节:直径11 mm-30 mm(体积>400 mm3)。
2.2.4 按数量[63-65]
①单发:单个病灶;②多发:2个及以上的病灶。
2.2.5 按危险因素[2,3,14,15]
①高危因素结节:年龄≥50岁且具有下列一种危险因素者:①吸烟≥20包年(或400年支),或曾经吸烟≥20包年(或400年支),戒烟时间<15年;②有环境或高危职业暴露史(如石棉、铍、铀、氡等接触者);③合并慢性阻塞性肺疾病、弥漫性肺纤维化或既往有肺结核病史者;④既往罹患恶性肿瘤或有肺癌家族史者;⑤低危因素结节:不具备上述危险因素者。近年来发现了许多年龄40岁-50岁、不吸烟、无环境或高危职业暴露史、不合并慢性阻塞性肺疾病、无弥漫性肺纤维化的女性表现为GGN样的肺癌患者[3-5,66-68]。其原因并不清楚,可能是与雌激素-受体介导的信号通路促进了女性肺腺癌的发生有关[69],也不排除部分中国非吸烟女性有长期被动吸烟史(香烟、烹饪烟雾)这一潜在影响因素[70]。按照结节大小和密度分类是最常用的分类方法,也是本共识的主要分类方法。
3. CT影像学评估
3.1 CT检查参数及测量
3.1.1 扫描参数[14,71-74]
CT是诊断肺部GGN的首选方法,强调薄层低剂量高分辨率CT扫描、靶扫描或靶重建,不需要注射对比剂。CT扫描探测器≥16排,扫描准直层厚:①建议1 mm薄层重建。如扫描层厚>1 mm,重建间隔选择准直层厚的50%-80%。重建图像矩阵512×512(最好选择1,024×1,024);②总辐射暴露剂量为1.0 mSv,120 kV,mAs≤40;③窗宽窗位:推荐肺窗窗位为-700 HU-600 HU,窗宽为1,500 HU-1,600 HU。纵隔窗窗位为30 HU-70 HU,窗宽为350 HU-400 HU;④扫描范围:深吸气末扫描,从肺尖到肋膈角,扫描采样时间≤10 s。CT筛选推荐低剂量扫描,在发现GGN后,推荐应用常规剂量进行靶扫描,以更精准地评估肺部GGN的结构。
3.1.2 测量及观察[75,76]
有关肺部GGN的大小、体积、密度、形态、边缘、内部结构、增长等情况的测量及观察,是制定肺部GGN处理策略的最关键技术指标。本共识基于实用性、可操作性、可重复性和有一定循证医学证据等原则达成如下共识:①测量单位:所有尺寸参数应精确至mm或mm3;②结节大小:肺窗下横截面最大长径;③结节体积:根据对结节的分割结果和结节所包含体素的数量计算结节体积;④结节实性成分测量:采用肺窗和纵隔窗相结合的方法,以肺窗测量为主(测量最大横截面长径)[77-79];⑤实性成分与肿瘤比率(consolidation tumor ratio,CTR)计算:肺窗下结节的横截面实性成分最大长径与结节最大长径之比;⑥密度、形态、边缘、内部结构:采用肺窗和纵隔窗相结合的方法,同时可以在不同轴位上观察,必要时三维重建;⑦体积倍增时间(volume-doubling time,VDT):VDT是判断GGN良恶性的重要参数之一[80-82],一般良性病变≥800 d,浸润前或微浸润病变为400 d-600 d,浸润性病变<400 d,传统意义上肺癌为100 d-300 d;⑧人工智能(artificial intelligence, AI)技术[83,84]:鉴于目前AI软件之间差异较大,如果在同一个医疗机构、用同型号CT扫描、同一种处理软件包,并且持续在该医疗机构长期随访复查,AI结果有较大的参考价值。
3.2 CT影像学评估
目前临床影像学上尚无评判GGN良恶性的统一标准,临床上常根据肺部GGN的影像学特征如大小、形态、边缘、瘤-肺界面、内部结构特征、位置及随访的动态变化来预测,其中以结节大小、内部结构特征(特别是实性成分)和随访的动态变化最为重要[79,85,86]。
3.2.1 GGN大小
①微小结节:直径<5 mm(体积<100 mm3),95%-99%为良性病变;②小结节:直径5 mm-10 mm(体积100 mm3-400 mm3),80%-85%为良性病变或浸润前病变;③结节:直径11 mm-30 mm(体积>400 mm3),如果经过3个月-4个月观察随访,不消失或不缩小并持续存在的GGN,60%-80%为浸润前病变或浸润性病变[63,82,87-91]。
3.2.2 形态
大多数恶性GGN的形态为圆形或类圆形,靠近叶间裂或大血管旁的恶性GGN出现不规则形态的比例较高。
3.2.3 边缘[92-94]
如果GGN呈分叶状(以浅分叶多见),或有毛刺征、胸膜凹陷征及血管集束征等征象常提示恶性的可能。炎性GGN边缘多模糊甚至有渗出样改变,良性非炎性肺结节边缘多整齐光滑。如果GGN边缘有尖角或纤维条索或周围出现纤维条索、胸膜增厚等征象则常提示结节为良性。
3.2.4 内部结构特征
①CT值[95-101]:目前认为当CT值>-450 HU在病理上通常是浸润性病变,但是由于GGN的面积较小,测量数值重复性较差,临床应用价值尚不能肯定;②CTR:mGGN中的实性成分是决定预后的主要因素,如果mGGN≥15 mm、CTR≥25%时在病理上通常是浸润性病变。CTR的增加或GGN整体增加或两者同步增加都预示着与浸润性肺癌的风险高度相关[79,102-10 4];③结节其他征象:空泡征、支气管充气征、血管在结节内扭曲或扩张等征象均提示GGN倾向于浸润性腺癌[105-107]。
3.3 随访的动态变化
发现GGN后随访是必须采取的措施。40%-50%的GGN在随访3个月-4个月后消失,这些消失的GGN多考虑是炎症[55,108-110]。将随访3个月-4个月后消失的称为暂时性GGN,反之称为持续性GGN。持续性GGN有潜在恶性的可能,这种潜在恶性的GGN要经过较长时间的发展才可能演变成为恶性。因此发现GGN要采用“观察-等待”(watchful-waiting)的方法进行一定时间的随访,观察其动态变化,以便确定GGN的性质[111-114]。pGGN和mGGN随访策略有所不同,但是在随访过程中出现如下情况多考虑为恶性:①病灶最大径或体积增大,VDT符合肿瘤生长规律;②病灶稳定或增大,并出现实性成分;③病灶稳定,但实性成分增加;④出现其他恶性征象:如分叶征、毛刺征、胸膜凹陷征、支气管充气征、血管集束征及血管在结节内扭曲或扩张等征象。随访过程中GGN发生增大和实性成分变化是核心指标,可参考图1进行随访和处理。
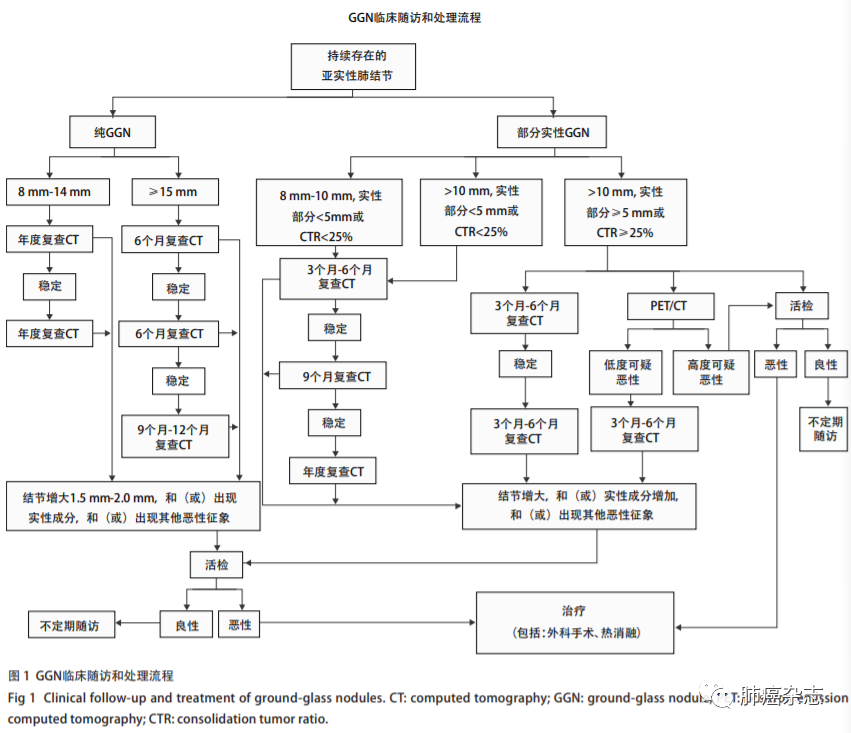
3.4 多发GGN
多发GGN是指肺内存在两个或以上最大径均≤30 mm的GGN,占肺部GGN的40% -50%。多发GGN有同侧肺同肺叶内多发、同侧肺不同肺叶内多发、双侧肺不同肺叶内单发或多发等多种类型。多发GGN主要病理类型为肺腺癌相关的组织亚型,涵盖了从AAH到AIS再到MIA最后到IAC等多个腺癌发展状态,甚至还可出现良恶性共存的情况[58, 63,115 -119]。由于多发GGN的多样性和复杂性,其处理手段仍未达成统一的共识[65,120 -122]。目前研究认为多发GGN每个病灶是“独立的个体”而非转移病灶[63-65,123-125],对于多发GGN的处理应遵循先“主”后“次”的原则,即先处理主病灶,再处理次病灶[31]。主病灶依据最大病灶来确定,但有时也用高度怀疑恶性的病灶来确定。多发GGN的预后取决于主病灶大小和实性成分,次要或残留病灶是否生长或是否有新发病灶一般不影响预后[65,124,126-128]。
4. 正电子发射型计算机断层显像(positron emissioncomputed tomography, PET)-CT
功能显像是进一步协助区分GGN良恶性的重要方法之一,但是PET-CT对GGN病变的诊断价值有限[129-134]。①pGGN:无论大小均不推荐PET-CT检查;②长径≤10 mm、实性成分<5 mm的mGGN,不推荐PET-CT;③长径11 mm-15 mm、实性成分≥5 mm的mGGN,推荐PET-CT进行定性 ,但存在较高的假阴性;④长径>15 mm、实性成分≥5 mm的mGGN,定性困难时推荐PET-CT,阳性率较高;⑤伴有肺内其他实性结节,或者有肺外恶性肿瘤病史的GGN患者,推荐行PET-CT检查;⑥PET-CT还可为选择穿刺活检部位提供重要依据。
5. 生物标志物
病理穿刺活检是明确GGN性质和决定治疗方式重要手段,经胸壁肺穿刺活检技术(percutaneous thoracic needlebiopsy, PTNB)和经支气管镜活检是最常用的两种非手术活检技术。
5.1 PTNB
5.1.1 PTNB适应证[135-144]
在CT引导下进行,参照图1。pGGN:①最大径< 8 mm不主张活检;②最大径8 mm-14 mm如果在随访过程中增大或出现实性成分;③最大径≥15 mm或在随访过程中增大或出现实性成分。mGGN:①最大径<8 mm,实性部分<5 mm或CTR<25%不主张活检;②最大径8 mm-10 mm,实性部分<5 mm或CTR<25%如果在随访过程中增大或实性成分增多;③最大径>10 mm(实性部分<5 mm或CTR<25%)在随访过程中增大或实性成分增多;④最大径>10 mm(实性部分≥5 mm或CTR ≥25%)如果在随访过程中增大或实性成分增多;⑤最大径>10 mm(实性部分≥5 mm或CTR ≥25%),PET-CT检查高度怀疑恶性。
5.1.2 PTNB禁忌证
PTNB除不可纠正的凝血功能障碍外绝对禁忌证很少[135,137]。相对禁忌证:①严重恶病质、严重心肺功能不全;②严重慢性阻塞性肺疾病、肺气肿、肺纤维化;③严重肺动脉高压;④机械通气(呼吸机)患者;⑤发作期精神病患者。
5.1. 3 PTNB诊断准确率[145 -148]
①直径≤8 mm的肺结节准确率70%-75%;②直径9 mm-10 mm的肺结节诊断准确率为80%-85%;③直径11 mm-20 mm的肺结节诊断准确率为85%-95%;④PTNB与手术后腺癌各亚型符合率约55%-60%[144,149]。
5.1.4 PTNB辅助技术
①消融后活检[150-156]:PTNB术中出现肺实质出血是影响诊断准确率的主要因素,微波消融或射频消融可以凝固肺内2 mm左右的小血管,消融后再取活检能减少肺实质出血,提高活检的阳性率。具体技术操作参考有关文献[150-154];②3D模板技术[157,158]:PTNB尤其是针对下叶GGN的活检存在一定难度,应用3D打印共面坐标模板联合固定针技术可以使GGN相对固定而减小呼吸运动对活检的影响,提高活检的阳性率。
5.2 气管镜检查
传统技术包括气管镜直视下刷检、活检或透视下经支气管镜肺活检及支气管肺泡灌洗获取细胞学和组织学对GGN的诊断价值不大。其他新发展的技术包括支气管内超声引导下肺活检术(endobronchial ultrasoundguided transbronchial needle aspiration, EBUS-TBLB)、虚拟导航气管镜(virtual bronchoscopic navigation, VBN)、电磁导航气管镜(electromagnetic navigation bronchoscopy,ENB)。采用可活检的超细气管镜,在其引导下超细气管镜可进入到第5-8级支气管用于GGN的活检[159-161]。
6. 局部热消融技术及影像引导方式
6.1 局部热消融技术
肿瘤热消融是针对某一脏器中特定的一个或多个肿瘤病灶,利用热产生的生物学效应直接导致病灶组织中的肿瘤细胞发生不可逆损伤或凝固性坏死的一种治疗技术。目前用于GGN治疗的主要包括射频消融(radiofrequency ablation, RFA)、微波消融(microwaveablation, MWA)和冷冻消融(cryoablation)。
6.1.1 RFA
RFA是目前治疗实体瘤最广泛的消融技术,其原理是将射频电极穿刺入肿瘤组织中,在375 kHz-500 kHz的高频交变电流作用下,肿瘤组织内的离子相互磨擦、碰撞而产生热生物学效应,局部温度可达60 oC-120 oC,当组织被加热至60 oC以上时,可引起细胞凝固性坏死。RFA消融体积取决于局部射频消融产生的热量传导与循环血液及细胞外液间的热对流,易受组织特性的影响[32,33,162] 。
6.1.2 MWA
MWA一般采用915 MHz或2,450 MHz两种频率。在微波电磁场的作用下,肿瘤组织内的水分子、蛋白质分子等极性分子产生极高速振动,造成分子之间的相互碰撞、相互摩擦,在短时间内产生高达60 oC-150 oC的高温,从而导致细胞凝固性坏死。由于辐射器将微波能集中在一定范围内,故而能有效地辐射到所需靶区,微波热辐射在肺内有更高的对流性和更低的热沉降效应[32,33,162]。
6.1.3 冷冻消融
常用的冷冻消融技术包括氩-氦冷冻消融和液氮冷冻系统。氩-氦冷冻消是通过焦耳-汤姆逊(JouleThomson)效应,高压氩气可以使靶组织冷却至-140 oC,氦气可使靶组织从-140 oC迅速上升至20 oC-40 oC。液氮冷冻消融可以使靶组织冷却至-196 oC,用无水乙醇升温至80 oC,通过这种温度梯度的变化可以导致[32,33,162]:①靶组织蛋白质变性;②细胞内外渗透压改变和“结冰”效应造成细胞裂解;③微血管栓塞引起组织缺血坏死等。用CT或磁共振成像(magnetic resonance imaging, MRI)观察到的“冰球”可以直接将消融区域与肿瘤边界进行区分,可以测定冷冻损伤的边界。 上述3种技术目前在治疗GGN方面均有应用[43-47,163,164],但是由于肺脏和GGN具有相对特殊的组织结构,MWA热辐射在肺内有更高的对流性和更低的热沉降效应,因此MWA对于治疗GGN具有一定的优势[41,46,47]。
6.2 影像引导技术
由于GGN影像学上的特殊性,CT是GGN消融治疗最常用的影像引导技术。穿刺时建议CT扫描层厚2.0 mm-2.5 mm,在肺窗下或合适的窗宽和窗位操作。
7. 热消融治疗肺GGN的适应证和禁忌证
肺癌患者的预后主要取决于是否存在肺门、纵隔淋巴结转移和远处转移。GGN样腺癌是肺腺癌的特殊亚型,主要是局部缓慢生长,而且有着不同的发展阶段,在AAH、AIS和MIA阶段几乎不出现淋巴结和远处转移。即便是IAC阶段,如果最大径≤30 mm、CTR≤50%也极少出现淋巴结和远处转移[20-27]。因此,热消融作为局部治疗的有效方法之一,完全可以通过热生物学效应治疗GGN,并且能够实现治愈性消融。治愈性消融是指通过热消融治疗,使局部肿瘤组织完全坏死,有可能达到治愈效果[32,165,166]。
7.1 适应证
7.1.1 周围型GGN患者
①因心肺功能差或高龄不能耐受手术切除;②拒绝行手术切除;③外科切除后又新出现的病灶或遗留病灶,患者无法耐受再次手术或拒绝再次手术;④多发GGN(先消融主病灶,其他病灶根据发展情况考虑再次消融);⑤各种原因导致的重度胸膜粘连或胸膜腔闭锁;⑥单肺(各种原因导致一侧肺缺如);⑦重度焦虑,经心理或药物治疗无法缓解。上述患者需经活检病理证实为AAH、AIS和MIA,对于周围型GGN样IAC患者要排除远处转移。
7.1.2 临床上常遇到几种既拒绝活检又拒绝手术的特殊患者
①有高危因素,影像学上有恶性征象(如病灶≥15 mm、毛刺征、分叶征、胸膜凹陷、空泡征、血管集束征、动态观察GGN增大、出现实性成分或实性成分增加等);②虽然没有高危因素,但是影像学上有恶性征象(如病灶>15 mm、毛刺征、分叶征、胸膜凹陷、空泡征、血管集束征、动态观察GGN增大、出现实性成分或实性成分增加等);③发现GGN后极度紧张和焦虑,经心理或药物治疗无法缓解[10, 31,167-169]。对于上述3种患者建议:首先多学科会诊(multidisciplinary team, MDT)共同讨论做出初步诊疗意见,在MDT的基础上与患者共同决策(shared decisionmaking, SDM)制定最终诊疗意见[170-173]。如果SDM意见是:“可不取病理直接消融或消融与活检同步进行”,那么医疗人员和患者及其家属(或监护人等)最终可按照SDM意见执行。SDM[174,175]是指在进行医疗和护理决策时,医务人员首先充分告知患者及其家属(或监护人等)各种诊疗措施的利弊、潜在的益处和风险,患者及其家属(或监护人等)通过权衡这些利弊,与医务人员充分沟通,最后共同做出决策。SDM是循证医学的重要内容之一,并且作为一种新型医疗模式,越来越受到关注。
7.2 禁忌证
7.2.1 绝对禁忌证
①血小板<50×109/L;②有严重出血倾向、短期内不能纠正的凝血功能障碍(凝血酶原时间>18 s,凝血酶原活动度<40%);③严重的肺纤维化和肺动脉高压;④抗凝治疗和(或)抗血小板药物在消融前停用未超过5 d-7 d,贝伐珠单抗末次使用间隔未超过1个月。
7.2.2 相对禁忌证
①胸腔积液控制不佳者;②肝、肾、心、肺、脑功能严重不全者;③严重贫血、脱水及营养代谢严重紊乱,无法在短期内纠正或改善者;④严重全身感染、高热(>38.5 oC)者;⑤美国东部肿瘤协作组(EasternCooperative Oncology Group, ECOG)评分>3分者;⑥发作期精神病患者;⑦合并其他肿瘤并有广泛转移者,预期生存期<6个月;⑧植入心脏起搏器的患者使用RFA时要在充分评估患者心功能的情况下,可考虑RFA手术期间停止起搏器,手术后恢复起搏器。
8. 术前准备
8.1 患者的评估及影像学检查
要通过认真复习病史、体格检查及近期的影像资料来评估患者的热消融适应证。适应证的选择建议MDT多学科(胸外科、肿瘤科、呼吸科、放射治疗科、介入医学科、影像科、病理科等)共同讨论做出决定,必要时进行SDM。胸部薄层CT(层厚≤1 mm,1个月内,可以不用强化)为消融治疗前评估的关键影像学检查,通过CT观察GGN的大小、形态、内部结构、位置及其与邻近重要脏器、血管、气管或支气管的关系。完善相关检查,如高度怀疑是肺部GGN处于Ia阶段可以在消融前行PET-CT检查或全身其他检查排除或发现远处转移。
8.2 各项实验室检查
实验室检查项目应包括:血常规、大小便常规、凝血功能、肝肾功能、血糖、肿瘤标志物、血型、血清传染病学等检查,心电图、肺功能、心脏彩超(高龄患者可选)等。
8.3 病理检查
在GGN消融前可行PTNB或行各种纤维支气管镜活检以明确诊断。
8.4 药品及监护设备准备
术前应准备麻醉、镇痛、镇咳、止血、扩血管、降压等药物,抢救药品及设备。
8.5 患者准备
①患者及(或)家属(被委托人)签署知情同意书,要充分告知患者及其家属(或监护人等)各种诊治方法潜在的获益和风险,积极鼓励参与SDM;②局部麻醉前4 h禁食,全身麻醉前12 h禁食、前4 h禁水;③手术区必要时备皮、建立静脉通道、术前口服镇咳剂和必要的镇静药物;④患者术前教育,主要是呼吸训练。
9. 麻醉与消毒
根据患者的状况,可以采用全身麻醉或局部麻醉进行消融手术[32]。穿刺点处用1%-2%利多卡因局部浸润麻醉,直至胸膜。对于术中不能配合、预计手术时间长、肿瘤贴近壁层胸膜可能引起较严重疼痛的患者,建议全身麻醉。严格执行无菌操作技术规范。
10. 消融操作
选择合适的消融技术后,CT是最常用和最准确的影像引导方式,操作过程是将热消融针在CT引导下通过皮肤直接精准地穿刺入靶组织中进行消融。消融的操作流程见图2。
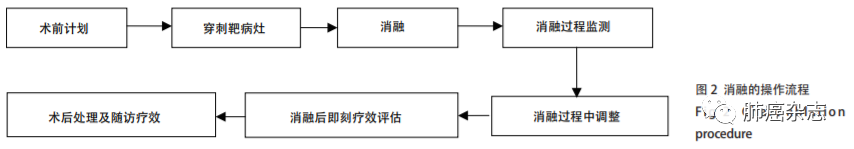
10.1 术前治疗计划
术前治疗计划是保证消融是否成功的关键环节。主要包括:①确定病变区域(gross tumor region,GTR):指影像学能界定的病变区域,即确定病灶的位置、大小、形态、与邻近器官的关系,初步确定GTR;②选择合适体位及穿刺点的体表定位;③穿刺路径:指从穿刺点到达病灶的穿刺通道,此距离称为“靶皮距”;④初步制定消融参数。
10.2 穿刺临床靶区
麻醉后用消融针按照术前计划的GTR,从体表定位点沿着穿刺路径逐层穿刺,分步进针,穿刺深度为术前计划的“靶皮距”,然后CT扫描观察(可通过三维重建影像确认)消融针是否到达预定的消融靶区。
10.3 消融靶组织
根据肿瘤的大小和部位可采用多种模式进行靶组织消融治疗:①单次单点完成消融;②单次多点完成消融;③多针单次多点;④对于多发病灶多点单次(每次消融≤3个病灶)或多次多点(双肺病灶间隔15 d左右)完成消融。所使用的消融参数(温度、功率、时间、循环等)根据不同的设备进行不同选择。
10.4 消融过程中监测
在消融过程中要监测消融针是否脱靶、是否需要调整消融针、是否达到了预定消融范围、是否有术中并发症(如出血、气胸)。热消融过程中,由于热消融对GGN周围肺组织的损伤,在GGN周围可出现不透明高密度区,称为消融后GGO,当GTR周围的GGO大于消融前GTR边界5 mm-10 mm时,消融针可以拔出。此时的靶组织定义为:消融后靶区(post-ablation target zone, PTZ)。消融过程需要监测心率、血压和血氧饱和度,同时要观察患者的呼吸、疼痛、咳嗽、咯血等情况,必要时应对症处理。
10.5 即刻疗效评价
①初步评价操作技术的成功情况;②观察消融边界建议:如果要达到完全消融,PTZ周围的消融后GGO至少要大于消融前GTR边界5 mm;③观察是否有并发症的发生。
10.6 术后处理
术后建议监测生命体征,24 h-48 h后CT扫描:①观察消融范围;②观察是否有并发症的发生(如无症状性气胸或胸腔积液)。
11. 随访及疗效评估
11.1 随访
术后1个月复查胸部CT,3个月后再复查胸部CT,主要观察局部病灶是否完全消融以及并发症等。以后每6个月复查胸部CT,主要观察局部病灶是否复发、是否逐渐形成疤痕、肺内是否有新发病灶等。两年后改为年度复查CT。
11.2 术后影像学特征及疗效评估
11.2.1 CT疗效评估
11.2 .1.1 影像学表现[32 ,176]
消融后由于消融区周围的出血、水肿、渗出、炎性细胞的浸润,PTZ显著大于原病灶的GTR,而这种影像学特征将持续3个月-4个月,因此传统的实体瘤疗效评价标准(Response Evaluation Criteria in SolidTumors, R ECIST)不适合用于消融后局部疗效的评价,特别是GGN。消融后CT扫描显示的变化规律为:消融后1个月-3个月内病灶增大,3个月后病灶保持稳定或逐渐缩小。①早期改变(1周内):可分为3层:a.内层:病灶内可出现实性或低密度泡影样改变;b.中间层:围绕着消融病灶周边形成的消融后GGO,一般认为GGO应超出肺结节周边边缘至少5 mm可达到肺GGN完全消融;c.外层:在GGO外有一层密度稍高于GGO的反应带。这种典型的影像学改变称为:“帽徽(cockade)”征象或“煎蛋(fried egg)”征(此征象在消融后24 h-48 h更加明显);②中期(1周-3个月内):消融区可持续增大,消融后GGO消失,其周边可能出现环绕清晰锐利的强化环,称为“蛋壳”(egg shell)征象;③后期(3个月后):与基线(一般以消融后4周-6周时的CT表现为基线)比PTZ在消融治疗3个月后病灶保持稳定,随后的CT随访过程中病灶区域有几种不同的演变模式: 如消失、缩小纤维化、空洞、结节、肺不张、增大(可能增生纤维化)等。冷冻消融术后的影像学变化特征与射频和微波消融相比有一定的差异,但可以参考上述变化过程。
11.2.1.2 局部疗效评估[32,162,176]
以消融后4周-6周时的病灶为基线判断疗效。①完全消融(出现下列表现任何一项):病灶消失、完全形成空洞、病灶纤维化(可为疤痕)、实性结节缩小或无变化或增大(但CT扫描无造影剂异常强化征象)、肺不张(肺不张内的病灶CT扫描无造影剂异常强化征象);②不完全消融(出现下列表现任何一项):a.在形成空洞形成边缘、在病灶纤维化边缘仍有典型的GGN影像学表现;b.病灶部分纤维化仍存有部分实性成分,且实性部分CT扫描强化和(或)PET-CT肿瘤有代谢活性;c.实性结节,大小无变化或增大,且伴CT扫描造影剂有异常强化征象和(或)PET-CT结节有异常代谢活性。
11.3 临床疗效评估
在判断局部疗效的基础上,定期随访评价临床疗效[32,162]。①技术成功和安全性评价至少随访6个月;②初步临床疗效评价至少随访1年;③中期临床疗效评价至少随访3年-5年;④长期临床疗效评价至少随访6年-10年。
12. 并发症及处理
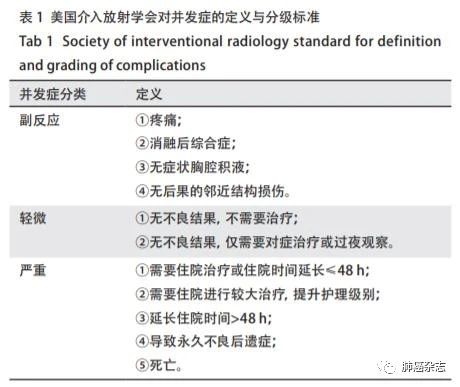
肺结节消融术是一种相对安全的局部治疗手段,其并发症的发生情况,依据美国介入放射学会(Societ y ofInterventional Radiology, SIR)的标准[177]进行评估分级。按照发生时间分为即刻并发症(消融后<24 h)、围手术期并发症(消融后24 h-30 d)及迟发并发症(消融后>30 d)(表1)。
12.1 不良反应
12.1.1 疼痛
在局麻条件下消融,一般均有不同程度的疼痛(尤其是邻近胸膜的疾病)。如果疼痛剧烈,可以加大阿片类止痛药物的用量,同时可以给予适量镇静剂。手术后疼痛一般为轻度疼痛,可持续数天,也有人持续1周-2周,很少出现中度以上的疼痛,可以用非甾体类药物止痛。
12.1.2 消融后综合征
约1/3的患者可能发生,是由于坏死物质的吸收和炎性因子的释放引起。主要症状为低热、乏力、全身不适、恶心、呕吐等,一般持续3 d-5 d。这种情况对症处理即可,必要时除给予非甾体类药物外,可以适量短时应用小剂量糖皮质激素。
12.1.3 咳嗽
消融术中出现咳嗽是十分常见的症状,剧烈的咳嗽可导致或加重气胸或皮下气肿,有时可使消融针移位。轻度的咳嗽不影响消融手术,剧烈咳嗽要停止消融手术或间断消融。引起咳嗽的原因可能与消融时局部温度增高刺激肺泡、支气管内膜或胸膜所致,术后咳嗽是肺结节组织坏死及其周围肺组织热损伤引起的炎症反应所致。术前1 h口服可待因可减轻咳嗽反应。术后咳嗽可适当给予止咳化痰药以及必要的抗生素。
12.1.4 胸膜反应
消融过程中刺激了支配壁层胸膜的迷走神经,兴奋的迷走神经可使心率减慢,甚至心跳停止。出现这种情况要暂停消融,要充分局部麻醉,并适当应用阿托品、镇静剂等药物。
12.2 并发症
12.2.1 气胸
气胸是消融后最常见的并发症,发生率为50%左右。气胸更常见于以下情况:肺气肿、男性、年龄>60岁、结节位于肺下叶、单发结节穿刺肺组织次数>3次、多发肺GGN消融多个结节穿刺次数多、穿过叶间裂、消融与活检同步或序贯进行。大部分气胸容易治疗,或者是自限性的,不需要治疗即可自愈,需要胸腔闭式引流的15%。如果患者经过胸腔闭式引流仍然有气体漏出,可以持续负压吸引、行胸膜固定术、气管镜下注入硬化剂、气管内置入阀门、胸腔镜手术修补等。另外,要注意迟发性气胸的发生。
12.2.2 胸腔积液
消融后经常可以见到少量胸腔积液,发生率为30%,被认为是机体对热损伤的交感反应,需要穿刺或置管引流的胸腔积液占5%。导致胸腔积液发生的危险因素包括大病灶、一次消融多个病灶、病灶靠近胸膜、消融时间长等。
12.2.3 出血
消融中出血的发生率为3%-8%,出血表现为咯血、血胸、失血性休克和急性呼吸衰竭,但主要表现为咯血和血胸。①咯血:出现中等以上的咯血时应立即消融病灶,同时静脉输注止血药。由于消融本身可以使血液凝固,随着消融治疗的进行出血会逐渐停止,故在具体消融治疗过程中大出血的发生率并不高。术后咯血多具有自限性,可持续3 d-5 d。保守治疗无效者,可行介入栓塞治疗或剖胸探查;②血胸:主要是因为在穿刺过程中损伤了胸廓内动脉、肋间动脉或其他动脉等。在穿刺过程中要避免穿刺到上述动脉,如果出现血胸要密切观察、积极治疗,保守治疗无效者,可行介入栓塞治疗或剖胸探查。
12.2.4 感染
对于老年并伴有重度基础肺部疾病的患者,肺部感染的机会更多,术前30 min-1 h可以预防性应用抗生素,24 h内再用一次。在下列情况下消融手术后预防性应用抗生素可以适当延长到48 h-72 h:老年人>70岁、长期慢性阻塞性肺气肿、糖尿病控制欠佳、单侧肺GGN消融数量>3个、免疫力低下等。若消融手术后5 d体温仍然>38.5 oC,首先要考虑肺部感染,要根据痰液、血液或脓液培养的结果调整抗生素。如果发生肺部或胸腔脓肿可以置管引流并冲洗。
12.2.5 空洞形成
空洞形成是肺部肿瘤热消融后的常见征象,可以视为术后的自然转归过程,但是也可能成为感染、出血等严重并发症的根源。大多术后15 d-1个月出现,2个月-4个月后吸收。大部分空洞没有症状,不需处理。如果出现发热、咳浓痰,应考虑空洞感染、脓肿形成,脓肿要及时引流。另外,要警惕曲霉菌感染。
12.2.6 其他
少见并发症:支气管胸膜瘘、非靶区热灼伤或冻伤、肋骨骨折、冷休克、血小板降低、神经损伤(臂丛、肋间、膈、喉返神经等)、肺栓塞、空气栓塞、心包填塞等需个别特殊处理。
13:结语
随着社会经济的发展、科学技术的进步和国民收入的增加,人们对健康保健需求提出了更高的标准,不但要有强壮的身体,还要有良好的心理状态和社会活动能力。根据人们对健康保健的新需求,传统的以诊疗“疾病”为中心的“生物医学”模式势必要转变为以“人”为中心的“生物-心理-社会”医学模式。LDCT筛查和由MDT讨论形成的对GGN处理意见作为典型的“生物医学”产物,一方面在发现早期肺癌和降低肺癌死亡率方面发挥了积极作用,另一方面也给人们带来了一系列的心理、社会和经济问题。因此要从“生物-心理-社会”多维度去正确认识LDCT筛查和MDT讨论意见,要把SDM融合到GGN的整个诊疗过程中,使患者获益最大化且风险最小化。另外,LDCT只是肺癌筛查的一项影像学技术,生物标志物与影像学联合筛查模式,可能更有助于肺癌的早期诊断,因此寻找敏感性高和特异性高的生物标志物是今后癌症筛查的方向之一。AI和云端技术是推动大数据创新应用的重要手段,也是医疗卫生健康领域未来发展趋势[178]。AI+云端技术形成的“物联网医学”[179]将有助于GGN同质化管理、多学科专家远程会诊和随访。
目前肺部GGN仍以局部治疗为主,热消融做为局部微创治疗技术之一,虽然在治疗GGN(尤其对于多发GGN)方面有一定优势,但是还存在许多问题:①缺乏大规模的、多中心的、前瞻性的临床研究;②缺乏长期(10年以上)临床疗效的随访结果;③缺乏与其他传统治疗手段(如VATS)的前瞻性的、随机的、多中心的临床比较研究;④如何精准定位,提高活检阳性率和局部完全消融率,是今后工作的方向之一;⑤电磁导航下肺GGN热消融技术也在发展,同时也显示了一定优势,但是普及该技术可能比较困难;⑥作为我国的“限制性医疗技术”,由于治疗设备的生产厂家不同,设备性能之间的差异,再加上该专业刚刚兴起,治疗人员的专业化水平参差不齐,现在很难形成公认的治疗规范和标准;⑦基础研究相对滞后,如复杂热场分布等;⑧人们对热消融技术治疗GGN还存在一定疑问,需要进一步开展工作以改变传统思维对热消融技术的认知,使得该技术得以普及和应用。
热消融治疗肺部亚实性结节专家共识(2021年版)
叶欣 范卫君 王忠敏 王俊杰 王徽 王俊 王春堂 牛立志 方勇 古善智 田辉 刘宝东 仲楼 庄一平池嘉昌 孙锡超 阳诺 危志刚 李肖 李晓光 李玉亮 李春海 李岩 杨霞 杨武威 杨坡 杨正强肖越勇 宋晓明 张开贤 陈仕林 陈炜生 林征宇 林殿杰 孟志强 赵晓菁 胡凯文 柳晨 柳澄顾春东 徐栋 黄勇 黄广慧 彭忠民 董亮 蒋磊 韩玥 曾庆师 靳勇 雷光焰 翟博 黎海亮 潘杰
中国医师协会肿瘤消融治疗技术专家组 中国医师协会介入医师分会肿瘤消融专业委员会中国抗癌协会肿瘤消融治疗专业委员会 中国临床肿瘤学会消融专家委员会
作者单位:250014 济南,山东第一医科大学第一附属医院(山东省千佛山医院)肿瘤中心,山东省肺癌研究所(叶欣,王俊,危志刚,李岩);510050 中山,中山大学肿瘤防治中心微创介入科(范卫君);200025 上海,上海交通大学医学院附属瑞金医院放射介入科(王忠敏);100191 北京,北京大学第三医院放射治疗科(王俊杰);170412 长春,吉林省肿瘤医院介入治疗中心(王徽);253022 德州,德州市第二人民医院胸外科(王春堂);510665 广州,暨南大学附属复大肿瘤医院肿瘤科(牛立志);310016 杭州,浙江大学医学院附属邵逸夫医院肿瘤内科(方勇);410013 长沙,湖南省肿瘤医院介入科(古善智);250012 济南,山东大学齐鲁医院胸外科(田辉);100053 北京,首都医科大学宣武医院胸外科(刘宝东);226001 南通,南通大学附属医院胸外科(仲楼);210009 南京,江苏省肿瘤医院介入治疗科(庄一平);200127 上海,上海交通大学医学院附属仁济医院肿瘤介入科(池嘉昌,翟博);250021 济南,山东第一医科大学附属省立医院病理科(孙锡超);530021 南宁,广西医科大学第一附属医院心胸外科(阳诺);100021 北京,中国医学科学院肿瘤医院介入治疗科(李肖,杨正强,韩玥);100730 北京,北京医院微创治疗中心(李晓光);250033 济南,山东大学第二医院介入医学科(李玉亮);250012 济南,山东大学齐鲁医院放射科(李春海);250101 济南,山东第一医科大学附属省立医院肿瘤中心(杨霞,黄广慧);100071 北京,解放军总医院第五医学中心肿瘤科(杨武威);150001 哈尔滨,哈尔滨医科大学附属第四医院介入血管外科(杨坡);100036 北京,中国人民解放军总医院放射诊断科(肖越勇);250014 济南,山东第一医科大学第一附属医院(山东省千佛山医院)胸外科(宋晓明);277500 滕州,山东滕州市中心人民医院肿瘤科(张开贤);210009 南京,江苏省肿瘤医院胸外科(陈仕林);350011 福州,福建医科大学附属肿瘤医院胸外科(陈炜生);350005 福州,福建医科大学附属第一医院介入科(林征宇);250021 济南,山东第一医科大学附属省立医院呼吸与危重症医学科(林殿杰);200032 上海,复旦大学附属肿瘤医院肿瘤微创治疗中心(孟志强);200127 上海,上海交通大学医学院附属仁济医院胸外科(赵晓箐);100078 北京,北京中医药大学附属东方医院肿瘤科(胡凯文);100161 北京,北京肿瘤医院介入治疗科(柳晨);250021 济南,山东省医学影像研究所CT研究室(柳澄);116011 大连,大连医科大学附属第一医院胸外科(顾春东);310022 杭州,中国科学院大学附属肿瘤医院(浙江省肿瘤医院)超声医学科(徐栋);250117 济南,山东第一医科大学附属肿瘤医院影像科(黄勇);250021 济南,山东第一医科大学附属省立医院胸外科(彭忠民);250014 济南,山东第一医科大学第一附属医院(千佛山医院)呼吸与危重症医学科(董亮);214063 无锡,华东疗养院放射科(蒋磊);250014 济南,山东第一医科大学第一附属医院(千佛山医院)医学影像科(曾庆师);215004 苏州,苏州大学附属第二医院介入治疗科(靳勇);710061 西安,陕西省肿瘤医院胸外科(雷光焰);450003 郑州,河南省肿瘤医院微创介入治疗科(黎海亮);100730 北京,中国医学科学院北京协和医院放射科(潘杰)(通讯作者:叶欣,E-mail: yexintaian2020@163.com;范卫君,E-mail: fanwj@sysucc.org.cn)
1 LATEST GLOBA L CA NCER DATA: CA NCER BUR DEN R ISES TO 19.3 MILLION NEW CASES A ND 10.0 MILLION CA NCER DEATHS IN 2020. https://www.iarc.fr/faq/latest-global-cancer-data2020-qa/ [Last accessed on 2021 Apr 18]
2 National Lung Screening Trial Research Team, Aberle DR, Adams A M, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med, 2011, 365(5): 395-409. doi: 10.1056/NEJMoa1102873
3 He YT, Zhang YC, Shi GF, et al. Risk factors for pulmonary nodules in North China: A prospective cohort study. Lung Cancer, 2018, 120: 122-129. doi: 10.1016/j.lungcan.2018.03.021
4 Yang W, Qian F, Teng J, et al. Community-based lung cancer screening with low-dose CT in China: Results of the baseline screening. Lung Cancer, 2018, 117: 20-26. doi: 10.1016/j.lungcan.2018.01.003
5 Fan L, Wang Y, Zhou Y, et al. Lung cancer screening with low-dose CT: baseline screening results in Shanghai. Acad Radiol, 2019, 26(10): 1283-1291. doi: 10.1016/j.acra.2018.12.002
6 Xu GH, Huang HX, Chen B, et al. A study on the first chest low-dose CT screening and susceptible factors of pulmonary nodules in 23,695 physical examinees in a medical examination center. Fudan Xue Bao (Yi Xue Ban), 2020, 47(5): 654-659. [徐国厚, 黄海峡, 陈斌, 等. 单体检中 心23,695例体检者首次胸部低剂量CT筛查结果及肺结节相关易感因素的研究. 复旦学报(医学版), 2020, 47(5): 654-659.]
7 Ca l l ister M E , Ba ldw i n DR , A k ra m A R , et al. Br it ish Thoracic Society u ide l i ne s f or t he i nv e s t i g at ion a nd m a n a ge me nt of pulmonary nodules. Thorax, 2015, 70 Suppl 2: ii1-ii54. doi: 10.1136/ thoraxjnl-2015-207168
8 Liu Y, Luo H, Qing H, et al. Screening baseline characteristics of early lung cancer on low-dose computed tomography with computer-aided detection in a Chinese population. Cancer Epidemiol, 2019, 62: 101567. doi: 10.1016/j.canep.2019.101567
9 Bach PB, Mirk in J N, Oliver TK, et al. Benef its and harms of CT screening for lung cancer: a systematic review. JAMA, 2012, 307(22): 2418-2429. doi: 10.1001/jama.2012.5521
10 Zhao Y, Wang R, Chen HQ. Progressions on diagnosis and treatment of ground-glass opacity. Zhongguo Fei Ai Za Zhi, 2016, 19(11): 773-777. [赵 悦, 王瑞, 陈海泉. 肺部磨玻璃影的诊断与治疗进展. 中国肺癌杂志, 2016, 19(11): 773-777.] doi: 10.3779/j.issn.1009-3419.2016.11.09
11 Kaaks R, Delorme S. Lung cancer screening by low-dose computed tomography - part 1: expected benefits, possible harms, and criteria for eligibility and population targeting. Rofo, 2021, 193(5): 527-536. doi: 10.1055/a-1290-7926
12 Bellinger C, Pinsky P, Foley K, et al. Lung cancer screening benefits and harms stratified by patient risk: information to improve patient decision aids. Ann Am Thorac Soc, 2019, 16(4): 512-514. doi: 10.1513/AnnalsATS.201810-690RL
13 Clark SD, Reuland DS, Enyioha C, et al. Assessment of lung cancer screening program websites. JAMA Intern Med, 2020, 180(6): 824-830. doi: 10.1001/jamainternmed.2020.0111
14 Society of Respiratory Diseases Lung Cancer Group of Chinese Medical A ssociation, Chinese Lung Cancer A ssociation. Chinese ex per t consensus on diagnosis and treatment of lung nodules (2018 edition). Zhonghua Jie He He Hu Xi Za Zhi, 2018, 41(10): 763-771. [中华医学会 呼吸病学分会肺癌学组, 中国肺癌防治联盟. 肺结节诊治中国专家 共识(2018年版). 中华结核和呼吸杂志, 2018, 41(10): 763-771.]
15 He J, Li N, Chen WQ , et al. Guidelines for lung cancer screening and early diagnosis and treatment in China (2021, Beijing). Zhonghua Zhong Liu Za Zhi, 2021, 43(3): 243-268. [赫捷, 李霓, 陈万青, 等. 中国 肺癌筛查与早诊早治指南(2021, 北京). 中华肿瘤杂志, 2021, 43(3): 243-268.] doi: 10.3760/cma.j.cn112152-20210119-00060
16 Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg, 2018, 66(2): 81-90. doi: 10.1007/s11748-017-0878-6
17 Jiang Y, Su Z, Liang H, et al. Video-assisted thoracoscopy for lung cancer: who is the future of thoracic surgery? J Thorac Dis, 2020, 12(8): 4427-4433. doi: 10.21037/jtd-20-1116
18 Hernandez-Vaquero D, Vigil-Escalera C, Pérez-Méndez I, et al. Survival after thoracoscopic surgery or open lobectomy: systematic review and meta-analysis. Ann Thorac Surg, 2021, 111(1): 302-313. doi: 10.1016/ j.athoracsur.2020.05.144
19 Chen D, K a ng P, Tao S, et al. Cost-ef fec t iveness eva luat ion of robotic-assisted thoracoscopic surger y versus open thoracotomy and v ideo-assisted thoracoscopic surger y for operable non-small cell lung cancer. Lung Cancer, 2021, 153: 99-107. doi: 10.1016/ j.lungcan.2020.12.033
20 K a k inuma R , Nog uchi M, A shizawa K , et al. Natura l histor y of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol, 2016, 11(7): 1012-1028. doi: 10.1016/j.jtho.2016.04.006
21 Shigefuku S, Shimada Y, Hagiwara M, et al. Prognostic significance of ground-glass opacity components in 5-year survivors with resected lung adenocarcinoma. Ann Surg Oncol, 2021, 28(1): 148-156. doi: 10.1245/ s10434-020-09125-x
22 Wang Q , Jiang W, Wang L, et al. Treatment principle and surgical technique of pulmonary ground glass nodules. Zhonghua Zhong Liu Za Zhi, 2019, 41(1): 6-9. [王群, 蒋伟, 王琳, 等. 肺部磨玻璃结节的处理原则和共识及手术技巧. 中华肿瘤杂志, 2019, 41(1): 6-9.] doi: 10.3760/cma.j.issn.0253-3766.2019.01.002.
23 Meng Y, Liu CL, Cai Q , et al. Contrast analysis of the relationship between the HRCT sign and new pathologic classification in small ground glass nodule-like lung adenocarcinoma. Radiol Med, 2019, 124(1): 8-13. doi: 10.1007/s11547-018-0936-x
24 Zhang Y, Chen H. Commentary: Is sublobar resection enough for ground-glass opacit y-dominant lung adenocarcinoma? J T horac Cardiovasc Surg , 2020, S0 022-5223(20)32782-3. doi: 10.1016/ j.jtcvs.2020.09.126
25 Zhang Y, Fu F, Chen H. Management of ground-glass opacities in the lung cancer spectrum. Ann Thorac Surg, 2020, 110(6): 1796-1804. doi: 10.1016/j.athoracsur.2020.04.094
26 Lee HW, Jin KN, Lee JK, et al. Long-term follow-up of ground-glass nodules after 5 years of stability. J Thorac Oncol, 2019, 14(8): 1370-1377. doi: 10.1016/j.jtho.2019.05.005
27 Mironova V, Blasberg JD. Evaluation of ground glass nodules.Curr Opin Pulm Med, 2018, 24(4): 350-354. doi: 10.1097/MCP.0000000000000492
28 Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest, 2013, 143(5 Suppl): e369S-e399S. doi: 10.1378/chest.12-2362
29 Jiang GN, Chen C, Zhu YM, et al. Shanghai pulmonary hospital experts consensus on the management of ground-glass nodules suspected as lung adenocarcinoma (Version 1). Zhongguo Fei Ai Za Zhi, 2018, 21(3): 147-159. [姜格宁, 陈昶, 朱余明, 等. 上海市肺科医院磨玻璃结节早 期肺腺癌的诊疗共识(第一版). 中国肺癌杂志, 2018, 21(3): 147-159.] doi: 10.3779/j.issn.1009-3419.2018.03.05
30 Liu J, Liu XQ , Yan BD, et al. Pulmonary multiple nodules: benign or malignant? Chin Med J (Engl), 2018, 131(16): 1999-2001. doi: 10.4103/0366-6999.238136
31 Song Y, Yuan DM. Some considerations in the management of pulmonary nodules. Zhonghua Yi Xue Za Zhi, 2019, 99(2): 81-83. [宋勇, 袁冬梅. 肺 部结节处理中的几点思考. 中华医学杂志, 2019, 99(2): 81-83.] doi: 10.3760/cma.j.issn.0376-2491.2019.02.001
32 Ye X, Fan W, Wang H, et al. Ex pert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J Cancer Res Ther, 2018, 14(4): 730-744. doi: 10.4103/ jcrt.JCRT_221_18
33 C hu K F, D upuy DE . T her ma l ablat ion of t u mou rs: biolog ic a l mechanisms and advances in therapy. Nat Rev Cancer, 2014, 14(3): 199-208. doi: 10.1038/nrc3672
34 Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-sma l l-cel l lu ng ca ncer (NSCLC): E SMO Cl i n ica l Pract ice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2017, 28(suppl_4): iv1-iv21. doi: 10.1093/annonc/mdx222
35 Quirk MT, Lee S, Murali N, et al. Alternatives to surgery for early-stage non-small cell lung cancer: thermal ablation. Clin Chest Med, 2020, 41(2): 197-210. doi: 10.1016/j.ccm.2020.02.002
36 Zeng C, Lu J, Tian Y, et al. Thermal ablation versus wedge resection for stage I non-small cell lung cancer based on the eighth edition of the TNM Classification: a population study of the US SEER database. Front Oncol, 2020, 10: 571684. doi: 10.3389/fonc.2020.571684
37 Wu J, Bai H X, Chan L , et al. Sublobar resection compared w ith stereotactic body radiation therapy and ablation for early stage non-small cell lung cancer: A National Cancer Database study. J Thorac Cardiovasc Surg, 2020, 160(5): 1350-1357.e11. doi: 10.1016/j.jtcvs.2019.11.132
38 Ager BJ, Wells SM, Gruhl JD, et al. Stereotactic body radiotherapy versu s perc ut a neou s loca l t u mor ablat ion for ea rly-st age nonsmall cell lung cancer. Lung Cancer, 2019, 138: 6-12. doi: 10.1016/ j.lungcan.2019.09.009
39 Uhlig J, Ludwig JM, Goldberg SB, et al. Survival rates after thermal ablation versus stereotactic radiation therapy for stage I non-small cell lung cancer: a national cancer database study. Radiology, 2018, 289(3): 862-870. doi: 10.1148/radiol.2018180979
40 Shyn PB. Is image-guided thermal ablation ready for treatment of stage I non-small cell lung cancer? Radiology, 2018, 289(3): 871-872. doi: 10.1148/radiol.2018181905
41 Ni Y, Xu H, Ye X. Image-guided percutaneous microwave ablation of early-stage non-small cell lung cancer. Asia Pac J Clin Oncol, 2020, 16(6): 320-325. doi: 10.1111/ajco.13419
42 Wei YT. Applicable CT-guided percutaneous radiofrequency ablation (R FA) in the treatment of unresectable lung ground glass opacity (GGO). Ann Oncol , 2017, 28 Suppl 2: ii47.
43 Iguchi T, Hiraki T, Gobara H, et al. Percutaneous radiofrequency ablation of lung cancer presenting as ground-glass opacity. Cardiovasc Intervent Radiol, 2015, 38(2): 409-415. doi: 10.1007/s00270-014- 0926-x
44 Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J Vasc Interv Radiol, 2014, 25(3): 333-339. doi: 10.1016/j.jvir.2013.11.035
45 Yang X, Ye X, Lin Z, et al. Computed tomography-guided percutaneous microwave ablation for treatment of peripheral ground-glass opacitylung adenocarcinoma: A pilot study. J Cancer Res Ther, 2018, 14(4): 764-771. doi: 10.4103/jcrt.JCRT_269_18
46 Huang G, Yang X, Li W, et al. A feasibility and safety study of computed tomography-guided percutaneous microwave ablation: a novel therapy for multiple synchronous ground-glass opacities of the lung. Int J Hyperthermia, 2020, 37(1): 414-422. doi: 10.1080/02656736.2020.175 6467
47 Liu S, Zhu X, Qin Z, et al. Computed tomography-guided percutaneous cryoablation for lung ground-glass opacity: A pilot study. J Cancer Res Ther, 2019, 15(2): 370-374. doi: 10.4103/jcrt.JCRT_299_18
48 Koizumi T, Kobayashi T, Tanabe T, et al. Clinical ex perience of bronchoscopy-g u ided rad iof requenc y ablat ion for per iphera lty pe lung cancer. Case Rep Oncol Med, 2013, 2013: 515160. doi: 10.1155/2013/515160
49 Her tzanu Y, Ye X . Computed tomography-g uided percutaneous microwave ablation: A new weapon to treat ground-glass opacity-lung adenocarcinoma. J Cancer Res Ther, 2019, 15(2): 265-266. doi: 10.4103/ jcrt.JCRT_65_19
50 Austin JH, Müller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology, 1996, 200(2): 327-331. doi: 10.1148/ radiology.200.2.8685321
51 Baldwin DR, Callister ME, Guideline Development Group. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax, 2015, 70(8): 794-798. doi: 10.1136/ thoraxjnl-2015-207221
52 Z hou QH, Fa n YG, Wa ng Y, et al. C h i na nat iona l g u idel i ne of classi f icat ion, d iag nosis a nd t reat ment for lu ng nodu les (2 016 version). Zhongguo Fei Ai Za Zhi, 2016, 19(12): 793-798. [周清华, 范 亚光, 王颖, 等. 中国肺部结节分类、诊断与治疗指南(2016年版). 中 国肺癌杂志, 2016, 19(12): 793-798.] doi: 10.3779/j.issn.1009-3419.20 16.12.12
53 Lee HY, Choi YL, Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am J Roentgenol, 2014, 202(3): W224-W233. doi: 10.2214/AJR.13.11819
54 Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med, 2015, 192(10): 1208-1214. doi: 10.1164/rccm.201505-0990OC
55 Loverdos K , Fot iad is A , Kontog ia nn i C, et al. Lung nodu les: A comprehensive review on current approach and management. A nn Thorac Med, 2019, 14(4): 226-238. doi: 10.4103/atm.ATM_110_19
56 Henschke CI, Yankelevitz DF, Libby DM, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med, 2006, 355(17): 1763-1771. doi: 10.1056/NEJMoa060476
57 Kim H, Park CM, Woo S, et al. Pure and part-solid pulmonary groundglass nodules: measurement variability of volume and mass in nodules with a solid portion less than or equal to 5 mm. Radiology, 2013, 269(2): 585-593. doi: 10.1148/radiol.13121849
58 Yatabe Y, Borczuk AC, Powell CA. Do all lung adenocarcinomas follow a stepwise progression? Lung Cancer, 2011, 74(1): 7-11. doi: 10.1016/ j.lungcan.2011.05.021
59 Kobayashi Y, Mitsudomi T, Sakao Y, et al. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol, 2015, 26(1): 156-161. doi: 10.1093/annonc/mdu505
60 Detterbeck FC, Bolejack V, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: Background data and proposals for the classification of lung cancer with separate tumor nodules in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol, 2016, 11(5): 681-692. doi: 10.1016/j.jtho.2015.12.114
61 Gao J W, R izzo S, Ma LH, et al. Pulmonar y ground-glass opacity: computed tomog raphy feat u res, h istopat holog y a nd molec u la r pathology. Transl Lung Cancer Res, 2017, 6(1): 68-75. doi: 10.21037/ tlcr.2017.01.02
62 Travis W D, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol, 2015, 10(9): 1240-1242. doi: 10.1097/JTO.0000000000000663
63 McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med, 2013, 369(10): 910-919. doi: 10.1056/NEJMoa1214726
64 Heuvelmans M A, Walter JE, Peters R B, et al. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer, 2017, 113: 45-50. doi: 10.1016/j.lungcan.2017.08.023
65 Liu B, Ye X . Management of pulmonar y multifocal ground-glass nodules: How many options do we have? J Cancer Res Ther, 2020, 16(2): 199-202. doi: 10.4103/jcrt.JCRT_377_20
66 Jia Q , Chen H, Chen X, et al. Barriers to low-dose CT lung cancer screening among middle-aged Chinese. Int J Environ Res Public Health, 2020, 17(19): 7107. doi: 10.3390/ijerph17197107
67 Yang D, Liu Y, Bai C, et al. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett, 2020, 468: 82-87. doi: 10.1016/j.canlet.2019.10.009
68 Kobayashi Y, Ambrogio C, Mitsudomi T. Ground-glass nodules of the lung in never-smokers and smokers: clinical and genetic insights. Transl Lung Cancer Res, 2018, 7(4): 487-497. doi: 10.21037/tlcr.2018.07.04
69 Skjefstad K, Grindstad T, Khanehkenari MR, et al. Prognostic relevance of estrogen receptor α, β and aromatase expression in non-small cell lung cancer. Steroids, 2016, 113: 5-13. doi: 10.1016/j.steroids.2016.05.008
70 Yu Y W, Wang CP, Han YF, et al. Meta-analysis on related risk factors regarding lung cancer in non-smoking Chinese women. Zhonghua Liu Xing Bing Xue Za Zhi, 2016, 37(2): 268-272. [余艺文, 王传鹏, 韩耀风, 等. 中国非吸烟女性肺癌危险因素的Meta分析. 中华流行病学杂志, 2016, 37(2): 268-272.] doi: 10.3760/cma.j.issn.0254-6450.2016.02.024
71 Christe A, Szucs-Farkas Z, Huber A, et al. Optimal dose levels in screening chest CT for unimpaired detection and volumetry of lung nodules, with and without computer assisted detection at minimal patient radiation. PLoS One, 2013, 8(12): e82919. doi: 10.1371/journal. pone.0082919
72 Ludwig M, Chipon E, Cohen J, et al. Detection of pulmonary nodules: a clinical study protocol to compare ultra-low dose chest CT and standard low-dose CT using ASIR-V. BMJ Open, 2019, 9(8): e025661. doi: 10.1136/bmjopen-2018-025661
73 National Lung Screening Trial Research Team, Aberle DR, Adams A M, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med, 2011, 365(5): 395-409. doi: 10.1056/NEJMoa1102873
74 Ohno Y, Aoyagi K, Chen Q , et al. Comparison of computer-aided detection (CADe) capability for pulmonary nodules among standard-, reduced- and ultra-low-dose CTs with and without hybrid type iterative reconstruction technique. Eur J Radiol, 2018, 100: 49-57. doi: 10.1016/ j.ejrad.2018.01.010
75 Division of Cardiothoracic Medicine, Society of Radiology, Chinese Medical Association, China Institute of Food and Drug Control. Data Annotation and quality control of Thoracic CT pulmonary nodules (2018). Zhonghua Fang She Xue Za Zhi, 2019, 53(1): 9-15. [中国食品 药品检定研究院中华医学会放射学分会心胸学组. 胸部CT肺结节 数据标注与质量控制专家共识(2018). 中华放射学杂志, 2019, 53(1): 9-15.] doi: 10.3760/cma.j.issn.1005-1201.2019.01.004
76 G odoy MC , Od i sio EG , Tr uong M T, e t al. P u l mona r y nodu le management in lung cancer screening: A pictorial review of lung-RADS version 1.0. Radiol Clin North Am, 2018, 56(3): 353-363. doi: 10.1016/ j.rcl.2018.01.003
77 Yanagawa M, Tsubamoto M, Satoh Y, et al. Lung adenocarcinoma at CT with 0.25-mm section thickness and a 2,048 matrix: high-spatialresolution imaging for predicting invasiveness. R adiolog y, 2020, 297(2): 462-471. doi: 10.1148/radiol.2020201911
78 Yanagawa M, Kusumoto M, Johkoh T, et al. Radiologic-pathologic correlation of solid por tions on thin-section CT images in lung adenocarcinoma: a multicenter study. Clin Lung Cancer, 2018, 19(3): e303-e312. doi: 10.1016/j.cllc.2017.12.005
79 Suzuk i K , Koike T, A sakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical Ia lung cancer ( Japan Clinical Oncolog y Group 0201). J Thorac Oncol, 2011, 6(4): 751-756. doi: 10.1097/JTO.0b013e31821038ab
80 Revel MP, Merlin A, Peyrard S, et al. Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. AJR Am J Roentgenol, 2006, 187(1): 135-142. doi: 10.2214/ AJR.05.1228
81 Nietert PJ, Ravenel JG, Leue W M, et al. Imprecision in automated volume measurements of pulmonary nodules and its effect on the level of uncertainty in volume doubling time estimation. Chest, 2009, 135(6): 1580-1587. doi: 10.1378/chest.08-2040
82 Larici A R , Farchione A, Franchi P, et al. Lung nodules: size still matters. Eur Respir Rev, 2017, 26(146): 170025. doi: 10.1183/160006 17.0025-2017
83 Prayer F, Rohrich S, Pan J, et al. Kunstliche Intelligenz in der Bildgebung der Lunge [Artificial intelligence in lung imaging]. Radiologe, 2020, 60(1): 42-47. doi: 10.1007/s00117-019-00611-2
84 Ye W, Gu W, Guo X, et al. Detection of pulmonary ground-glass opacity based on deep learning computer artificial intelligence. Biomed Eng Online, 2019, 18(1): 6. doi: 10.1186/s12938-019-0627-4
85 Fu F, Zhang Y, Wen Z, et al. Distinct prognostic factors in patients with stage I non-small cell lung cancer with radiologic part-solid or solid lesions. J Thorac Oncol, 2019, 14(12): 2133-2142. doi: 10.1016/ j.jtho.2019.08.002
86 Cho J, Kim ES, Kim SJ, et al. Long-term follow-up of small pulmonary ground-glass nodules stable for 3 years: implications of the proper follow-up period and risk factors for subsequent growth. J Thorac Oncol, 2016, 11(9): 1453-1459. doi: 10.1016/j.jtho.2016.05.026
87 MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Societ y 2017. R adiolog y, 2017, 284(1): 228-243. doi: 10.1148/radiol.2017161659
88 Horeweg N, van Rosmalen J, Heuvelmans M A, et al. Lung cancer probabi lit y in patients w ith CT-detected pulmonar y nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol, 2014, 15(12): 1332-1341. doi: 10.1016/ S1470-2045(14)70389-4
89 Robbins HA, Katki HA, Cheung LC, et al. Insights for management of ground-glass opacities from the national lung screening trial. J Thorac Oncol, 2019, 14(9): 1662-1665. doi: 10.1016/j.jtho.2019.05.012
90 Oud kerk M, Liu S, Heuvel ma ns M A , et al. Lu ng ca ncer L DCT screening and mortality reduction - ev idence, pitfalls and f uture perspectives. Nat Rev Clin Oncol, 2021, 18(3): 135-151. doi: 10.1038/ s41571-020-00432-6
91 de Margerie-Mellon C, Gill R R, Monteiro Filho AC, et al. Growth assessment of pulmonary adenocarcinomas manifesting as subsolid no d u l e s o n C T: C o m p a r i s o n o f d i a me t e r - b a s e d a nd v o l u me measurements. Acad Radiol, 2020, 27(10): 1385-1393. doi: 10.1016/ j.acra.2019.09.028
92 Wu F, Tian SP, Jin X, et al. CT and histopathologic characteristics of lung adenocarcinoma with pure ground-glass nodules 10 mm or less in diameter. Eur Radiol, 2017, 27(10): 4037-4043. doi: 10.1007/ s00330-017-4829-5
93 Zhu Y, Hou D, Lan M, et al. A comparison of ultra-high-resolution CT target scan versus conventional CT target reconstruction in the evaluation of ground-glass-nodule-like lung adenocarcinoma. Quant Imaging Med Surg, 2019, 9(6): 1087-1094. doi: 10.21037/qims.2019.06.09
94 Matsunaga T, Suzuki K, Takamochi K, et al. What is the radiological definition of part-solid tumour in lung cancer? Eur J Cardiothorac Surg, 2017, 51(2): 242-247. doi: 10.1093/ejcts/ezw344
95 Xu DM , v a n K laveren R J, de Boc k GH , e t al. R ole of ba sel i ne nodule density and changes in density and nodule features in the discrimination between benign and malignant solid indeterminate pulmonary nodules. Eur J Radiol, 2009, 70(3): 492-498. doi: 10.1016/ j.ejrad.2008.02.022
96 Kobayashi H, Ohkubo M, Narita A, et al. A method for evaluating the performance of computer-aided detection of pulmonary nodules in lung cancer CT screening: detection limit for nodule size and density. Br J Radiol, 2017, 90(1070): 20160313. doi: 10.1259/bjr.20160313
97 Chu ZG, Li W J, Fu BJ, et al. CT cha rac ter ist ics for pred ic t i ng invasiveness in pulmonar y pure ground-glass nodules. AJR A m J Roentgenol, 2020, 215(2): 351-358. doi: 10.2214/AJR.19.22381
98 Lee HY, Choi YL, Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am J Roentgenol, 2014, 202(3): W224-W233. doi: 10.2214/AJR.13.11819
99 Zhou Q J, Zheng ZC, Zhu YQ , et al. Tumor invasiveness defined by I ASLC/ATS/ERS classification of ground-glass nodules can be predicted by quantitative CT parameters. J Thorac Dis, 2017, 9(5): 1190-1200. doi: 10.21037/jtd.2017.03.170
100 Z h a n Y, Peng X , S h a n F, e t al. A t tenu at ion a nd mor pholog ic characteristics disting uishing a ground-glass nodule measuring 5-10 mm in Diameter as invasive lung adenocarcinoma on thin-slice CT. AJR Am J Roentgenol, 2019, 213(4): W162-W170. doi: 10.2214/ AJR.18.21008
101 Niu R, Shao X, Shao X, et al. Lung adenocarcinoma manifesting as ground-glass opacity nodules 3 cm or smaller: evaluation with combined high-resolution CT and PET/CT modality. AJR Am J Roentgenol, 2019, 213(5): W236-W245. doi: 10.2214/AJR.19.21382
102 Zhang Y, Fu F, Wen Z, et al. Segment location and ground glass opacity ratio reliably predict node-negative status in lung cancer. Ann Thorac Surg, 2020, 109(4): 1061-1068. doi: 10.1016/j.athoracsur.2019.10.072
103 Tsurugai Y, Kozuka T, Ishizuka N, et al. Relationship between the consol idation to ma x imum t umor diameter ratio and outcomes fol low ing stereotactic body radiotherapy for stage I non-sma l lcel l lu ng c a ncer. Lu ng Ca ncer, 2 016 , 9 2: 47-52 . doi: 10 .1016/ j.lungcan.2015.12.003
104 Su H, Dai C, Xie H, et al. Risk factors of recurrence in patients with clinical stage Ia adenocarcinoma presented as ground-glass nodule. Clin Lung Cancer, 2018, 19(5): e609-e617. doi: 10.1016/j.cllc.2018.04.020
105 Wu FZ, Chen PA, Wu CC, et al. Semiquantative visual assessment of sub-solid pulmonar y nodules ≤3 cm in differentiation of lung adenocarcinoma spectrum. Sci Rep, 2017, 7(1): 15790. doi: 10.1038/ s41598-017-16042-9
106 Gao F, Su n Y, Zha ng G, et al. CT cha rac ter izat ion of d i f ferent pathological types of subcentimeter pulmonary ground-glass nodular lesions . Br J R ad iol, 2 019, 9 2(10 9 4): 2 018 0 2 0 4. doi: 10.1259/ bjr.20180204
107 Gao F, Li M, Ge X , et al. Mu lti-detector spira l CT st udy of the re l at ion s h ip s b e t w e e n pu l mon a r y g rou nd - g l a s s no du le s a nd blood vessels. Eur Radiol, 2013, 23(12): 3271-3277. doi: 10.1007/ s00330-013-2954-3
108 Libby DM, Wu N, Lee IJ, et al. CT screening for lung cancer: the value of short-term CT follow-up. Chest, 2006, 129(4): 1039-1042. doi: 10.1378/chest.129.4.1039
109 O h J Y, Kw o n S Y, Yo o n H I , e t a l . C l i n i c a l s i g n i f i c a n c e o f a solitar y ground-glass opacit y (GGO) lesion of the lung detected b y c he s t C T. L u ng C a nc er, 2 0 0 7, 55(1): 67-73 . doi: 10 .1016/ j.lungcan.2006.09.009
110 Felix L, Serra-Tosio G, Lantuejoul S, et al. CT characteristics of resolving ground-glass opacities in a lung cancer screening programme. Eur J Radiol, 2011, 77(3): 410-416. doi: 10.1016/j.ejrad.2009.09.008111
111 N a s i m F, O s t D E . M a n a g e m e n t o f t h e s o l i t a r y p u l m o n a r y nodule. Curr Opin Pulm Med, 2019, 25(4): 344-353. doi: 10.1097/ MCP.0000000000000586
112 Hanaoka T, Kurai M, Okada M, et al. Preoperative watchful-waiting time and surgical outcome of patients with non-small cell lung cancer found by chest low-dose CT screening. World J Surg, 2018, 42(7): 2164-2172. doi: 10.1007/s00268-017-4439-z
113 Seok Y, Cho S, Kim K, et al. Partly solid pulmonary nodules: waiting for change or surgery outright? Interact Cardiovasc Thorac Surg, 2014, 19(4): 556-560. doi: 10.1093/icvts/ivu205
114 G u l a t i C M , S c h r e i n e r A M , L i b b y D M , e t a l . O u t c o m e s o f u n resec ted g rou nd-g lass nodu les w it h c y tolog y suspicious for adenocarcinoma. J Thorac Oncol, 2014, 9(5): 685-691. doi: 10.1097/ JTO.0000000000000143
115 Heuvelmans M A, Walter JE, Peters R B, et al. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer, 2017, 113: 45-50. doi: 10.1016/j.lungcan.2017.08.023
116 Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest, 2013, 144(4): 1291-1299. doi: 10.1378/chest.12-2987
117 Detterbeck FC, Bolejack V, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: background data and proposals for the classification of lung cancer with separate tumor nodules in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol, 2016, 11(5): 681-692. doi: 10.1016/j.jtho.2015.12.114
118 Liu M, He WX, Song N, et al. Discrepancy of epidermal growth factor receptor mutation in lung adenocarcinoma presenting as multiple ground-glass opacities. Eur J Cardiothorac Surg, 2016, 50(5): 909-913. doi: 10.1093/ejcts/ezw113
119 Kobayashi Y, Mitsudomi T, Sakao Y, et al. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol, 2015, 26(1): 156-161. doi: 10.1093/annonc/mdu505
120 Sihoe A DL, Petersen R H, Cardillo G. Multiple pulmonary ground glass opacities: is it time for new guidelines? J Thorac Dis, 2018, 10(11): 5970-5973. doi: 10.21037/jtd.2018.10.67
121 Qu R, Hao Z, Zhang Y, et al. Single-center experience of simultaneous bilateral uni-portal video-assisted thoracoscopic surgery for multiple ground-glass opacities. J Cardiothorac Surg, 2020, 15(1): 69. doi: 10.1186/s13019-020-01107-0
122 Lococo F, Cusumano G, De Filippis AF, et al. Current practices in the management of pulmonary ground-glass opacities: a survey of SICT members. Ann Thorac Surg, 2018, 106(5): 1504-1511. doi: 10.1016/ j.athoracsur.2018.06.053
123 Hattori A, Matsunaga T, Takamochi K, et al. Surgical management of mu lt i foca l g round-glass opacit ies of t he lung: cor relat ion of clinicopathologic and radiologic findings. Thorac Cardiovasc Surg, 2017, 65(2): 142-149. doi: 10.1055/s-0036-1572437
124 Hattori A, Takamochi K, Oh S, et al. Prognostic classification of mu lt iple pr i ma r y lung cancers based on a g round-glass opacit y component. Ann Thorac Surg, 2020, 109(2): 420-427. doi: 10.1016/ j.athoracsur.2019.09.008
125 Taralli S, Scolozzi V, Foti M, et al. 18F-FDG PET/CT diagnostic performance in solitary and multiple pulmonary nodules detected in patients with previous cancer history: reports of 182 nodules. Eur J Nucl Med Mol I mag i ng , 2 019, 4 6(2): 429 - 436. doi: 10.10 0 7/ s00259-018-4226-6
126 Gu B, Bur t BM, Mer r itt R E , et al. A dominant adenocarcinoma with multifocal ground glass lesions does not behave as advanced d isease. A n n T horac Su rg , 2 013, 96(2): 411- 418. doi: 10.1016/ j.athoracsur.2013.04.048
127 K i m H K , C hoi YS , K i m J, et al. M a nagement of mu lt iple pu re ground-glass opacit y lesions in patients w ith bronchioloalveolar ca rcinoma. J T horac Oncol, 2010, 5(2): 206 -210. doi: 10.1097/ JTO.0b013e3181c422be
128 Liu BD. Diagnosis and treatment of pulmonary multifocal ground-glass nodules. Zhongguo Fei Ai Za Zhi, 2020, 23(8): 679-684. [刘宝东. 肺 多发磨玻璃结节的诊治策略. 中国肺癌杂志, 2020, 23(8): 679-684.] doi: 10.3779/j.issn.1009-3419.2020.102.10
129 Niyonkuru A, Bakari KH, Lan X. 18F-f luoro-2-deoxy-d-glucose PET/ computed tomography evaluation of lung cancer in populations with high prevalence of tuberculosis and other granulomatous disease. PET Clin, 2018, 13(1): 19-31. doi: 10.1016/j.cpet.2017.08.003
130 Chun EJ, Lee HJ, Kang WJ, et al. Differentiation between malignancy and inflammation in pulmonary ground-glass nodules: The feasibility of integrated 18F-FDG PET/CT. Lung Cancer, 2009, 65(2): 180-186. doi: 10.1016/j.lungcan.2008.11.015
131 Ruilong Z, Daohai X, Li G, et al. Diagnostic value of 18F-FDG-PET/ CT for the evaluation of solitary pulmonary nodules: a systematic review and meta-analysis. Nucl Med Commun, 2017, 38(1): 67-75. doi: 10.1097/MNM. 0000000000000605
132 Li W, Pang H, Liu Q , et al. The role of ¹⁸F-FDG PET or ¹⁸F-FDG-PET/ CT in the evaluation of solitary pulmonary nodules. Eur J Radiol, 2015, 84(10): 2032-2037. doi: 10.1016/j.ejrad.2015.06.008
133 Hochhegger B, Alves GR, Irion K L, et al. PET/CT imaging in lung cancer: indications and findings. J Bras Pneumol, 2015, 41(3): 264-274. doi: 10.1590/S1806-37132015000004479
134 Shao X, Niu R, Jiang Z, et al. Role of PET/CT in management of early lung adenocarcinoma. AJR Am J Roentgeno, 2020, 214(2): 437-445. doi: 10.2214/AJR.19.21585
135 Yoon SH, Lee SM, Park CH, et al. 2020 Clinical practice guideline for percutaneous transthoracic needle biopsy of pulmonary lesions: a consensus statement and recommendations of the Korean Society of Thoracic Radiology. Korean J Radiol, 2021, 22(2): 263-280. doi: 10.3348/kjr.2020.0137
136 Yu H, Zhang C, Liu S, et al. Application value of coa x ial biopsy system in needle cutting biopsy for focal ground glass-like density nodule. J Cancer Res Ther, 2018, 14(7): 1509-1514. doi: 10.4103/jcrt. JCRT_382_18
137 Chinese Anti-cancer Association Professional Committee on Cancer Intervention, China Anti-cancer Association Professional Committee on Breast Cancer. China expert consensus on percutaneous biopsy of Thoracic Tumor (2020 edition). Zhonghua Yi Xue Za Zhi, 2021, 101(3): 185-198. [中国抗癌协会肿瘤介入学专业委员会, 中国抗癌协会肿瘤 介入学专业委员会胸部肿瘤诊疗专家委员会. 胸部肿瘤经皮穿刺 活检中国专家共识(2020版). 中华医学杂志, 2021, 101(3): 185-198.] doi: 10.3760/cma.j.cn112137-20200907-02576
138 Han Y, Kim HJ, Kong KA, et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS One, 2018, 13(1): e0191590. doi: 10.1371/journal.pone.0191590
139 Yamagami T, Yoshimatsu R, Miura H, et al. Diagnostic performance of percutaneous lung biopsy using automated biopsy needles under CTf luoroscopic guidance for ground-glass opacity lesions. Br J Radiol, 2013, 86(1022): 20120447. doi: 10.1259/bjr.20120447
140 Sharma A, Shepard JO. Lung cancer biopsies. Radiol Clin North Am, 2018, 56(3): 377-390. doi: 10.1016/j.rcl.2018.01.001
141 Zhou Q , Dong J, He J, et al. The Society for Translational Medicine: indications and methods of percutaneous transthoracic needle biopsy for diagnosis of lung cancer. J Thorac Dis, 2018, 10(9): 5538-5544. doi: 10.21037/jtd.2018.09.28
142 Tsai PC, Yeh YC, Hsu PK, et al. CT-guided core biopsy for peripheral sub-solid pulmonary nodules to predict predominant histological and aggressive subtypes of lung adenocarcinoma. Ann Surg Oncol, 2020, 27(11): 4405-4412. doi: 10.1245/s10434-020-08511-9
143 Yamagami T, Yoshimatsu R, Miura H, et al. Diagnostic performance of percutaneous lung biopsy using automated biopsy needles under CTf luoroscopic guidance for ground-glass opacity lesions. Br J Radiol, 2013, 86(1022): 20120447. doi: 10.1259/bjr.20120447
144 Halpenny D, Das K, Ziv E, et al. Percutaneous computed tomography guided biopsy of sub-solid pulmonary nodules: differentiating solid from ground glass components at the time of biopsy. Clin Imaging, 2021, 69: 332-338. doi: 10.1016/j.clinimag.2020.07.011
145 Zheng Y F, Jia ng L M , M ao W M , et al. Perc uta neou s computed t omo g r aphy- g u ide d lu n g biop s y of s ol it a r y no du l a r g rou nd - glass opacity. J Cancer Res Ther, 2015, 11 Suppl: C231-C233. doi: 10.4103/0973-1482.162117
146 Ya ng JS , L iu Y M , M ao Y M , e t al. Me ta-a na ly si s of CT- g u ided transthoracic needle biopsy for the evaluation of the ground-glass opacity pulmonary lesions. Br J Radiol, 2014, 87(1042): 20140276. doi: 10.1259/bjr.20140276
147 Liu GS, Wang SQ , Liu HL, et al. Computed tomography-guided biopsy for small (≤20 mm) lung nodules: a meta-analysis. J Comput Assist Tomogr, 2020, 44(6): 841-846. doi: 10.1097/RCT.0000000000001071
148 Yamauchi Y, Izumi Y, Nakatsuka S, et al. Diagnostic performance of percutaneous core needle lung biopsy under multi-CT f luoroscopic guidance for ground-glass opacity pulmonary lesions. Eur J Radiol, 2011, 79(2): e85-e89. doi: 10.1016/j.ejrad.2011.03.088
149 Huang KY, Ko PZ, Yao CW, et al. Inaccuracy of lung adenocarcinoma subtyping using preoperative biopsy specimens. J Thorac Cardiovasc Surg, 2017, 154(1): 332-339.e1. doi: 10.1016/j.jtcvs.2017.02.059
150 Liu J, Huang W, Wu Z, et al. The application of computed tomographyguided percutaneous coaxial biopsy combined with microwave ablation for pulmonary tumors. J Cancer Res Ther, 2019, 15(4): 760-765. doi: 10.4103/jcrt.JCRT_778_18
151 Wang D, Li B, Bie Z , et al. Sy nchronous core-needle biopsy and microwave ablation for highly suspicious malignant pulmonary nodule via a coaxial cannula. J Cancer Res Ther, 2019, 15(7): 1484-1489. doi: 10.4103/jcrt.JCRT_721_18
152 Wang J, Ni Y, Yang X, et al. Diagnostic ability of percutaneous core biopsy immediately after microwave ablation for lung ground-glass opacity. J Cancer Res Ther, 2019, 15(4): 755-759. doi: 10.4103/jcrt. JCRT_399_19
153 Hasegawa T, Kondo C, Sato Y, et al. Pathologic diagnosis and genetic analysis of a lung tumor needle biopsy specimen obtained immediately after radiofrequency ablation. Cardiovasc Intervent Radiol, 2018, 41(4): 594-602. doi: 10.1007/s00270-017-1845-4
154 Hasegawa T, Kondo C, Sato Y, et al. Diagnostic ability of percutaneous needle biopsy immediately after radiofrequency ablation for malignant lung tumors: an initial experience. Cardiovasc Intervent Radiol, 2016, 39(8): 1187-1192. doi: 10.1007/s00270-016-1324-3
155 Li X, Ye X. Computed tomography-guided percutaneous core-needle biopsy after thermal ablation for lung ground-glass opacities: Is the method sound? J Cancer Res Ther, 2019, 15(7): 1427-1429. doi: 10.4103/ jcrt.JCRT_926_19
156 Kong F, Wang C, Li Y, et al. Advances in study of the sequence of lung tumor biopsy and thermal ablation. Thorac Cancer, 2021, 12(3): 279-286. doi: 10.1111/1759-7714.13795
157 Xu JM, Yu YC, Liu Z, et al. The application of 3D printed coplanar template combined with fixed needle technique in accurate percutaneous biopsy of small pulmonary nodules. Zhongguo Zu Zhi Gong Cheng Yan Jiu, 2021, 25(5): 761-764. [徐俊马, 喻岳超, 刘智, 等. 3D打印共面模板 结合固定针技术在肺小结节经皮精准活检中的应用. 中国组织工 程研究, 2021, 25(5): 761-764. ]
158 Ji Z, Wang G, Chen B, et al. Clinical application of planar puncture template-assisted computed tomog raphy-g u ided perc uta neous biopsy for small pulmonary nodules. J Cancer Res Ther, 2018, 14(7): 1632-1637. doi: 10.4103/jcrt.JCRT_1017_17
159 Bauer TL, Berkheim DB. Bronchoscopy: diagnostic and therapeutic for non-small cell lung cancer. Surg Oncol Clin N Am, 2016, 25(3): 481-491. doi: 10.1016/j.soc.2016.02.009
160 Epelbaum O, Aronow WS. Autof luorescence bronchoscopy for lung cancer screening: a time to reflect. Ann Transl Med, 2016, 4(16): 311. doi: 10.21037/atm.2016.06.34
161 Folch EE, Labarca G, Ospina-Delgado D, et al. Sensitivity and safety of electromagnetic navigation bronchoscopy for lung cancer diagnosis: systematic review and meta-analysis. Chest, 2020, 158(4): 1753-1769. doi: 10.1016/j.chest.2020.05.534
162 Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminolog y and reporting criteria--a 10 -year update. Radiology, 2014, 273(1): 241-260. doi: 10.1148/radiol.14132958
163 Kim KY, Jin GY, Han YM, et al. Cryoablation of a small pulmonary nodule with pure ground-glass opacity: a case report. Korean J Radiol, 2015, 16(3): 657-661. doi: 10.3348/kjr.2015.16.3.657
164 Zhang YS, Niu LZ , Zhan K , et al. Percutaneous imaging-g uided cryoablation for lung cancer. J Thorac Dis, 2016, 8(Suppl 9): S705-S709. doi: 10.21037/jtd. 2016.09.42
165 Liu BD, Ye X, Fan W J, et al. Ex per t consensus on image-g uided radiofrequency ablation of pulmonary tumors: 2018 edition. Thorac Cancer, 2018, 9(9): 1194-1208. doi: 10.1111/1759-7714.12817
166 Venturini M, Cariati M, Marra P, et al. CIRSE standards of practice on thermal ablation of primary and secondary lung tumours. Cardiovasc Intervent Radiol, 2020, 43(5): 667-683. doi: 10.1007/s00270-020-02432-6
167 Brain K, Lifford KJ, Carter B, et al. Long-term psychosocial outcomes of low-dose CT screening: results of the UK Lung Cancer Screening randomised controlled trial. Thorax, 2016, 71(11): 996-1005. doi: 10.1136/thoraxjnl-2016-208283
168 Gareen IF, Duan F, Greco EM, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer, 2014, 120(21): 3401-3409. doi: 10.1002/cncr.28833
169 Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest, 2018, 153(4): 1004-1015. doi: 10.1016/j.chest.2017.10.013
170 Austin CA, Mohottige D, Sudore R L, et al. Tools to promote shared decision making in serious illness: a systematic review. JAMA Intern Med, 2015, 175(7): 1213-1221. doi: 10.1001/jamainternmed.2015.1679
171 Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision making for lung cancer screening. JAMA Intern Med, 2018, 178(10): 1311-1316. doi: 10.1001/jamainternmed.2018.3054
172 Geerse OP, Stegmann M E, Kerstjens H A, et al. Effects of shared decision making on distress and health care utilization among patients with lung cancer: a systematic review. J Pain Symptom Manage, 2018, 56(6): 975-987.e5. doi: 10.1016/j.jpainsymman.2018.08.011
173 Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest, 2021: S0012-3692(21)00115-X. doi: 10.1016/j.chest.2021.041
174 Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns, 2015, 98(10): 1172-1179. doi: 10.1016/j.pec.2015.06.022
175 Rivera MP, Henderson LM. Lung cancer screening and shared decision making in cancer survivors: the long and winding road. Transl Lung Cancer Res, 2019, 8(2): 119-123. doi: 10.21037/tlcr.2018.12.14
176 Araujo-Filho JA, Menezes RS, Horvat N, et al. Lung radiofrequency ablation: post-procedure imaging patterns and late follow-up. Eur J Radiol Open, 2020, 7: 100276. doi: 10.1016/j.ejro.2020.100276
177 Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol, 2003, 14(9 Pt 2): S199-S202. doi: 10.1097/01.rvi.0000094584.83406.3e
178 Tian XQ , You M. Issues and policy suggestions on the development of artificial intelligence in medical institutions in China. Wei Sheng Ruan Ke Xue, 2019, 33(10): 42-44. [田雪晴, 游茂. 我国医学人工智能在 医疗机构发展面临的问题及政策建议. 卫生软科学, 2019, 33(10): 42-44.]
179 China Expert Group on Internet of things assisted diagnosis and treatment of pulmonary nodules. China expert consensus on Internet of things assisted diagnosis and treatment of pulmonary nodules. Guo Ji Hu Xi Za Zhi, 2017, 37(8): 561-568. [中国物联网辅助肺结节诊治专 家组. 物联网辅助肺结节诊治中国专家共识. 国际呼吸杂志, 2017, 37(8): 561-568.] doi: 10.3760/cma.j.issn.1673-436X.2017.08.001
|