|
Ⅲ期临床研究,入组标准为直径<2 cm 的部分实性磨玻璃样病变或实性结节,按医学中心、性别、组织学类型以及病变形态(部分实性磨玻璃样病变或实性结节)分层,随机分为肺叶切除组和肺段切除组,计划入组1100 例患者,主要预后终点为总体生存率,次要终点为手术后肺功能。
- Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe SI, Asamura H; West Japan Oncology Group and Japan Clinical Oncology Group. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022 Apr 23;399(10335):1607-1617. doi: 10.1016/S0140-6736(21)02333-3. PMID: 35461558. 5年OS,肺段优于肺叶
- Suzuki K , et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019 手术并发症相似
- Hattori A , et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer with radiologically pure-solid appearance in Japan (JCOG0802/WJOG4607L): a post-hoc supplemental analysis of a multicentre, open-label, phase 3 trial. Lancet Respir Med. 2024. 纯实性结节,RFS 肺叶优于肺段切除术,尤其是在<70岁,女性人群
- Maniwa T, et al. Lymph node dissection in small peripheral lung cancer: Supplemental analysis of JCOG0802/WJOG4607L. J Thorac Cardiovasc Surg. 2024. 淋巴结清扫:部分实性结节邻近叶间和纵隔淋巴结清扫不影响分期,实性结节至少应行选择性清扫
- Nakagawa K , et al. Risk Factors for Locoregional Relapse After Segmentectomy: Supplementary Analysis of the JCOG0802/WJOG4607L Trial. J Thorac Oncol. 2025. 纯实性结节、切缘距离小于肿瘤大小和男性与肺段切除术局部复发率高显著相关。
- Ryu Nakajima, et al. Long-Term Outcomes of Segmentectomy Versus Lobectomy in Small-Sized Peripheral Non-Small Cell Lung Cancer: A 10-Year Follow-Up Analysis of the Phase 3 Randomized Trial (JCOG0802/WJOG4607L). AATS, 2025. 10年OS,两组相似
- Akamine K, et al. Segmentectomy versus lobectomy in small-sized, peripheral, and pathologically invasive non-small cell lung cancer: Supplemental analysis of JCOG0802/WJOG4607L. AATS, 2025. 病理上有浸润性癌特征,肺段切除术局部复发率高,但生存至少不劣于肺叶切除术
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe SI, Asamura H; West Japan Oncology Group and Japan Clinical Oncology Group. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022 Apr 23;399(10335):1607-1617. doi: 10.1016/S0140-6736(21)02333-3. PMID: 35461558.
Background: Lobectomy is the standard of care for early-stage non-small-cell lung cancer (NSCLC). The survival and clinical benefits of segmentectomy have not been investigated in a randomised trial setting. We aimed to investigate if segmentectomy was non-inferior to lobectomy in patients with small-sized peripheral NSCLC.
Methods: We conducted this randomised, controlled, non-inferiority trial at 70 institutions in Japan. Patients with clinical stage IA NSCLC (tumour diameter ≤2 cm; consolidation-to-tumour ratio >0·5) were randomly assigned 1:1 to receive either lobectomy or segmentectomy. Randomisation was done via the minimisation method, with balancing for the institution, histological type, sex, age, and thin-section CT findings. Treatment allocation was not concealed from investigators and patients. The primary endpoint was overall survival for all randomly assigned patients. The secondary endpoints were postoperative respiratory function (6 months and 12 months), relapse-free survival, proportion of local relapse, adverse events, proportion of segmentectomy completion, duration of hospital stay, duration of chest tube placement, duration of surgery, amount of blood loss, and the number of automatic surgical staples used. Overall survival was analysed on an intention-to-treat basis with a non-inferiority margin of 1·54 for the upper limit of the 95% CI of the hazard ratio (HR) and estimated using a stratified Cox regression model. This study is registered with UMIN Clinical Trials Registry, UMIN000002317.
Findings: Between Aug, 10, 2009, and Oct 21, 2014, 1106 patients (intention-to-treat population) were enrolled to receive lobectomy (n=554) or segmentectomy (n=552). Patient baseline clinicopathological factors were well balanced between the groups. In the segmentectomy group, 22 patients were switched to lobectomies and one patient received wide wedge resection. At a median follow-up of 7·3 years (range 0·0-10·9), the 5-year overall survival was 94·3% (92·1-96·0) for segmentectomy and 91·1% for lobectomy (95% CI 88·4-93·2); superiority and non-inferiority in overall survival were confirmed using a stratified Cox regression model (HR 0·663; 95% CI 0·474-0·927; one-sided p<0·0001 for non-inferiority; p=0·0082 for superiority). Improved overall survival was observed consistently across all predefined subgroups in the segmentectomy group. At 1 year follow-up, the significant difference in the reduction of median forced expiratory volume in 1 sec between the two groups was 3·5% (p<0·0001), which did not reach the predefined threshold for clinical significance of 10%. The 5-year relapse-free survival was 88·0% (95% CI 85·0-90·4) for segmentectomy and 87·9% (84·8-90·3) for lobectomy (HR 0·998; 95% CI 0·753-1·323; p=0·9889). The proportions of patients with local relapse were 10·5% for segmentectomy and 5·4% for lobectomy (p=0·0018). 52 (63%) of 83 patients and 27 (47%) of 58 patients died of other diseases after lobectomy and segmentectomy, respectively. No 30-day or 90-day mortality was observed. One or more postoperative complications of grade 2 or worse occurred at similar frequencies in both groups (142 [26%] patients who received lobectomy, 148 [27%] who received segmentectomy).
Interpretation: To our knowledge, this study was the first phase 3 trial to show the benefits of segmentectomy versus lobectomy in overall survival of patients with small-peripheral NSCLC. The findings suggest that segmentectomy should be the standard surgical procedure for this population of patients.
亚肺叶切除与肺叶切除的效果比较。 直径≤2 cm 非小细胞肺癌患。 JCOG0802 研究中亚肺叶切除组仅纳入了肺段切除。 JCOG0802 研究结果显示与肺叶切除相比,肺段切除并没有增加围术期总并发症发生率;仅复杂肺段切除增加围术期支气管胸膜瘘发生率。
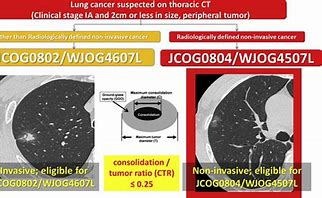
相关文献:
【评论】
JCOG 0802 VS CALGB 140503 VS LCSG
JCOG0802实性结节亚组分析_这篇文章告诉你什么病人应该做肺段切除
【相似研究】
参考文献:
- Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022 Apr 23;399(10335):1607-1617.
- Kenichi Nakamura, et al. A Phase III Randomized Trial of Lobectomy Versus Limited Resection for Small-sized Peripheral Non-small Cell Lung Cancer (JCOG0802/WJOG4607L)
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy vs limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-622.[Abstract/Free Full Text]
- Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study J Thorac Cardiovasc Surg 2006;132:769-775.[Abstract/Free Full Text]
- Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination Ann Thorac Surg 2009;88:1106-1111.[Abstract/Free Full Text]
|
|